-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2013; 3(5): 145-154
doi:10.5923/j.re.20130305.06
Speciation of Zinc and Copper in Open-Air Automobile Mechanic Workshop Soils in Ngara Area-Nairobi Kenya
Chengo Katana, Murungi Jane, Mbuvi Harun
Department of Chemistry, Kenyatta University P.O. Box 43844 00100 Nairobi, Kenya
Correspondence to: Mbuvi Harun, Department of Chemistry, Kenyatta University P.O. Box 43844 00100 Nairobi, Kenya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Increased imports of reconditioned motor vehicle in Kenya have led to an unprecedented mushrooming of open-air automobile mechanic garages in urban areas. Despite the toxic contaminants contained in garage waste, these open-air garageshave remained unregulated and theireffect to the surrounding soils and water bodies has not been evaluated. The primary objective of this study was to determine the concentration levels of Zn and Cuin the various fractions of garage soilsas a means of assessing their impacts on the environment. Soil samples were collected twice from ten sampling sites of the open-air automobile garages in Ngara area – Nairobi Kenya at a depth of 0 to 10cm. The modified Community Bureau of Reference (BCR) sequential extraction was used and metal concentration done usingFAAS. The total mean concentration levels of Zn and Cu were found to be3335.05±199.31mg/kg and 525.33±15.10mg/kg and ranged from 2962.42±754.15mg/kg to 3705.07±858.27mg/kg and 452.00±12.21mg/kg to 730.82±18.98mg/kg for Zn and Cu respectively. This indicates that the levels of the metals are high compared to the maximum allowed limits. The concentration levels of Znin the various soil fractions were almost evenly distributed in the four fractions. The percentage ratio of Zn in the fractions followed the order: reducible(26.95%)>residual(24.96%)>oxidisable(24.40%)>exchangeable(23.43%) while for Cu they followed the order: residual(51.23%)>oxidisable(27.27%)>reducible(11.31%)>exchangeable(10.19%). The sum of the percentage ratios of Zn and Cu found in the non-residual fractions was 75.04 and 48.77 respectively. The average of their mobility factors were23.93 and10.4andranged from 16.13 to 34.77 and 7.82 to 12.50 for Zn and Cu respectively. This shows that the concentrations of these metals in the soils were very high and substantial proportions of them were in the exchangeable fraction hence mobile and bioavailable. As expected from the high concentrations and mobilitiesobtained, the concentration of these metals in the nearby grass, water pools and runoffs were high.Further,their concentrationsupstream were found to belower than downstream, an indicationthat they were translocating from the garage to the surrounding environment. Pearson correlation of the concentration levels of these metalsin the exchangeable fraction with their total concentration in the soilgave significantly positive values of Zn (r=0.822) and Cu (r=0.457). This suggests that high metal concentration levels in the soils infer increased mobility and therefore bioavailability.
Keywords: Speciation, Mobility, Bioavailability, Sequential Extraction, Heavy Metals, Soil
Cite this paper: Chengo Katana, Murungi Jane, Mbuvi Harun, Speciation of Zinc and Copper in Open-Air Automobile Mechanic Workshop Soils in Ngara Area-Nairobi Kenya, Resources and Environment, Vol. 3 No. 5, 2013, pp. 145-154. doi: 10.5923/j.re.20130305.06.
Article Outline
1. Introduction
- Soils consist of a heterogeneous medium that comprises of decomposed rock fragments, clay minerals, oxides of Fe, Al and Mn, organic materials, organo-metallic complexes and soil solutions[1]. It plays an important role in sustaining life as the very survival of mankind is tied to its productivity[2]. Apart from being a medium for plants to grow, itcan also a transmit pollutants including potentially toxic metals into the atmosphere, biosphere and water resources[3]. Heavy metals in soil exist in several different forms and are associated with various components[4]. Their accumulation in soil, particularly Pb, Cd, Cu, Ni and Zn, is of concern due to their increased use in industrial processes and commercial products[5,6 ,7 ,8]. Heavy metals emanating from anthropogenic sources are of great concern due to their instability and solubility that makes them mobile and bioavailable[9,10]. They can enter the environment in a bioavailable form and are capable of transforming to other species that are more toxic[11]. They easily accumulate in the topsoil to toxic levels due to their persistence andnon-biodegradability[12,13] and eventually make their way to humans through the food chain, where they perturb biological processes[14]. Although Cu and Zn are essential elements, they are potentially toxic to crops, animals and humans at high concentration levels[15].It is a known fact that automobiles introduce a number of toxic metals into the environment[16]. Auto tire wear, degradation of parts and greases, peeling paint and metals in auto-catalysts are sources of heavy metal pollution[17]. Increased imports of reconditioned automobiles has led to increased wear and tear, mushrooming of open-air garageand elevatedconcentration levels of heavy metals in the soils[18,19 ,20]. Despite this, published data on heavy metal contamination in the soils is limited and the few available report total concentrations. These provide limited information on their potential toxic behaviour, mobility and bioavailability [21]. This is unacceptable especially because studies elsewhere have shown that automobile mechanic garages and automobile scrap yards harbour substantial amounts of heavy metals[5,6,22 ,23]. Once in soil,metals can exist in various forms classified as mobile, mobilisableand immobile fractions[14]. Therefore, the total metal concentration alone does not provide predictive insights on their bioavailability, mobility and fate[24,25]. The chemical forms of the metalsareimportant factors in assessing their impacts on theenvironment[26,27 ,28]. A high concentration and mobility level of these metals poses great danger to life and its environment asthey canmake their way to humans through the food chain[86]. There has been increased interest in the studies on speciation or chemical forms of heavy metals in polluted soils and sediments using sequential extraction techniques because these provide knowledge on metal affinity to soil components and the strength with which they are bound to matrix. The use of sequential extractions, although time consuming, furnishes detailed information about the origin, mode of occurrence, biological and physicochemical availability, mobilization and transport of themetals[29,30]. Sequential extraction procedures selectively extract metals bound by specific soil fractions with minimal effects on the soil components. In practice, sequential fractionation schemes have been suggested to identify element distribution within operationally defined soil pools[31]. These chemical pools range from water soluble to recalcitrant forms immobilized in mineral lattices. In the modified BCR, a four step sequential extraction method, has made it possible to harmonize the extraction schemes for the determination of extractable metals and can be used to enhance quality control of the whole analysis procedure, a key issue in interpreting this type of operational defined speciation.The procedure consists of using four fractions which allow the identification of three distinct parts (or compartments) and a residual: The Fraction bound to carbonates (F1) comprises metals adsorbed on the surface of soil. It is the most accessible and represents the exchangeable fraction. Metals on this fraction are easily mobile and are assumed to be available. The decrease of pH leads them to migrate from the solid phase to water and plants[36]. This represents the water and acid soluble as well as exchangeable fraction and is extracted with 0.11 molL-1 acetic acid[41]. The fraction of metal bound to iron and manganese oxides (F2) is sensitive to redox potential changes and represents the fraction that can be solubilized under reducible conditions. It represents the reducible fraction and extracted using a solution of 0.1 M NH2OH.HCl at pH 2. The fraction bound to organic matter (F3) is temporarily inaccessible and can only be solubilized under chemical oxidation. It represents the fraction combined with organic materials and is extracted using a solution of 1.0 M NH4Ac after the sample has been digested with H2O2[32,41]. The Residual fraction (F4) mainly contains metals built in the crystal lattice of minerals. In natural conditions, they are practically inaccessible for living organisms and can be considered as permanently immobile. This fraction is extracted using aqua regia.The aim of this study was to determine the concentration levels of Zn and Cu in the various fractionsof soils of open-air automobile mechanic garage of Ngara, Nairobi, Kenya so as to assess their association, bioavailability and environmental contamination risk based on their chemical form. The need for this research arose becausethere are no reports on speciation of heavy metals in soil of these garages despite their rapid increase.
2. Materials and Methods
2.1. Area of Study
- Nairobi, the capital city of Kenya covers an area of 697 km2and has a population of over 3.1 million[33]. Nationally, Nairobi has been established to have the greatest concentration of industrial and automobile pollution sources[34]. The main focus of the study was Ngara area located within Nairobi city–Kenya. The area is a host to many open-air vehicle mechanic workshops. A section of the Nairobi river cuts across the study area from northwest to southeast.
2.2. Sampling and Sample Pre-treatment
- Ten sampling points each 4m x 4m quadrants were chosen with reference to potential sources of the heavy metals. Each quadrant was subdivided into twenty cells (20cm by 20cm) denoting a sampling point. Soil samples from ten randomly selected cells of each quadrant were collected at depths of 0-10cm and mixed to obtain a representative composite sample. This was done twice in the rainy month of April ten days apart. The samples obtained were placed in separate labelled polyethylene bags and transported to the laboratory at room temperature on the same day. In the laboratory extraneous materials were removed, the soil samples air-dried and homogenized by grinding. They were then oven-dried to a constant mass for 48 hours at 50℃, cooled and sieved using size 600 µm mesh to remove large undesired particles sizes. The sieved soil samples were then ground to powder form using an agate mortar and pestle to particle sizes of nanometres range. The resultant powder was stored in clean labelled polyethylene bags in a desiccator until analysis[37].
2.3. Analysis
- Heavy metals in the soil were extracted using the modified BCR chemical sequential extraction[40,41]. Theexchangeable and acid soluble fractions (F1), was extracted by shaking about 1.0g soil sample in a 40mL solution of 0.11 M CH3COOH for 16 hours at room temperature. The reducible fractions (F2), was extracted by shaking the residue from (F1) in a 40mL solution of 0.1 M NH2OH.HCl at pH 2 for 16 hours at room temperature.The residue from (F2) was treated twice with 8.8 molL-1 hydrogen peroxide, evaporated to near dryness, 50 mL of ammonium acetate was added and the pH was adjusted to 2 using nitric acid and shaken overnight. The mixture was then centrifuged to separate (F3), the extract representing the oxidizable fraction. The residual fraction was extracted by digesting residue from (F3) using 20mL of aqua regiasolution[40,41]. Similarly extract for total metal concentration (bulk soil analysis) was obtained by extracting about 1.0g of soil sample with about 20mL of aqua regiasolution[40,41]. The concentration of metal in the various fractions was determined using a flame atomic absorption spectrophotometer (Model Buck Scientific210 VGP). The levels of Cr and Ni obtained in the various fraction and bulk were used to calculate their concentrations in the fractions and bulk soil respectively.The recovery of the sequential extraction was obtained as a percentage ofthe sum of four fractions over directly measured total, [(sum of fractions/total metal)x100][35].All analysis was done in triplicate and the three absorbance readings averaged. Quality checks were also performed on the instrument by checking the absorbance after every ten sample runs[36].The pH and total organic carbon were measured using standard procedures[37]. Soil pH was measured by using a suspension of 1.0g of soil placed in 10mL of deionized water. For total organic carbon (T O C), 1.0 g of soil (dried at 105℃ for 1hr) was placed in a ceramic crucible, heated to 550℃ for 2 hours, cooled in a desiccator then weighed[38]. The soil organic matter (SOM) obtained was converted to total organic carbon (TOC) using a 1.9 correctionfactor[39,40 ,41]. The measurements were done in triplicates. All chemicals were of analytical grade and all plastic and glass wares for metal analysis were previously soaked in 10%nitric acid (HNO3) (v/v), for 48 hours to remove all entrained metals, washed with detergents and rinsed with deionized water. All glassware were soaked in 10% HNO3, washed before use, and rinsed with deionized water. The arithmetic means (AM) of the triplicate extraction results were calculatedand their standard deviations (STD) determined[42].
3. Results and Discussion
3.1. Total Heavy Metal Concentration
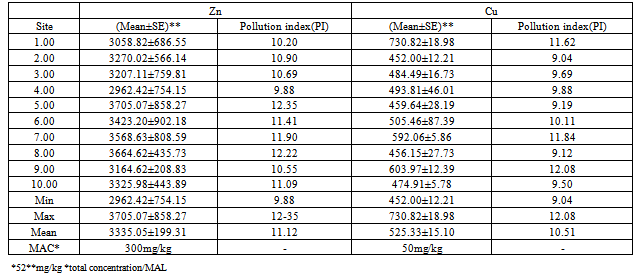
- The total concentration levels of the metals are shown in table 1 and their variations at the ten sampling sites expressed as a bar graph in Fig 1. Themean of the total concentrations of zinc and copper were 3335.05 ± 199.31mg/kg and 525.33±15.10mg/kg and ranged from 2962.42±754.15mg/kg to 3705.07±858.27mg/kg and from 452.00±12.21mg/kg to730.82±18.98mg/kg respectively. These concentration levels exceeded the warning and critical limits for Zn in soil, which are set at 150 and 300 mg/kgrespectively[43,44 ,45] and the maximum allowed concentrations for Cu in soils set at 50 mg/kg[46]. The high concentration levels and high standard deviation values point to a spatial distribution attributed to conditions either inherited from human interferences with nature and/or induced by various sources of contaminations at the area[47]. This supports that they are derived from the automobile repair activities at the study area.The contamination index (pollution index-Pi) of the metals in the soil samples was obtained by dividing the total concentration obtained by its maximum allowed limit, MAL[48]. The mean pollution indexes obtained were11.12 for Zn and 10.51 for Cu as shown in Table 1. Traditionally the data obtained from calculation of the contamination index is grouped into four grades that range from unpolluted to highly polluted soils as follows: 0 to 0.99 (uncontaminated), 1.0 to 1.19 (moderately to highly contaminated), 1.2 to 1.99 (highly contaminated), 2.0 to 3.5 (very highly contaminated)[10,49]. Based on this scale, it is clear that the study site is highly contaminatedwith the heavy metals studied.
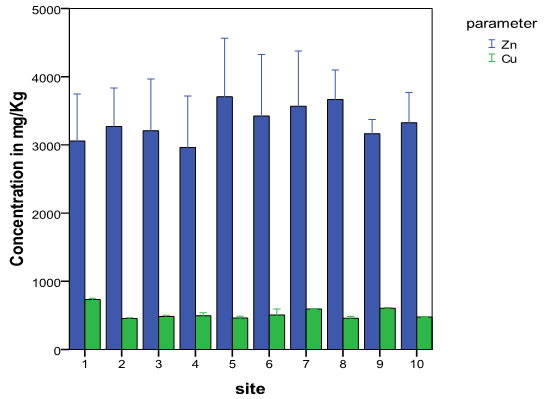 | Figure 1. Variations in the total concentration of Zn and Cu at the ten sites |
|
|
3.2. Fractionation of the Metals
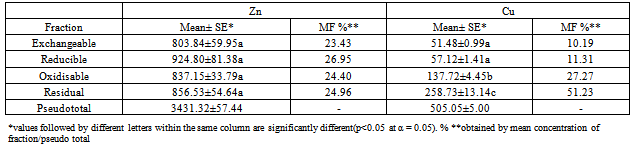
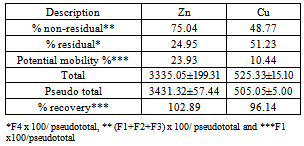
- The average concentration levels of Zn and Cu in the four soil fractions at the ten sites areshowninTable2. From the results, Zn though almost evenly distributed in the four fractions was highest in the reducible fraction with about 27% of the total concentration in the soil while the exchangeable fraction had the lowest with about 23%. The concentration levels in the fractions followed the order: Reducible (924.80 ± 81.38mg/kg)> residual(856.53±54.64mg/kg) > oxidisable (837.15 ± 33.79mg/kg) > exchangeable (803.84 ± 59.95mg/kg). This is in agreement with earlier workers that have reported Zn to be preferably associated with Fe-Mn oxides[50,51]. Zinc in this form (reducible fraction) has been reported to be an important source of heavy metals under reducing conditions[52]. Studies have also shown that a high proportion of metalsin association with this oxide phases is indicative of anthropogenic pollution[4,48,49]. ForCu, the residual fraction had the highest concentration level of about 51% of the total while the exchangeable had the lowest at about 10%. The concentration levels in the fractions followed the order: Residual (258.73 ± 13.14mg/kg) > oxidisable (137.72 ± 4.45mg/kg) > reducible (57.12 ± 1.41mg/kg) > exchangeable (51.48±0.99mg/kg).The moderately high percentage of Cu in the oxidisable (organic bound) fraction indicates its strong ability of the heavy metal to form complexes with organic matter thereby reducing its mobility and phytotoxicity[53]. Comparable percentagesof theorganic-bound Cu that ranges between 28 to 32% of the total metal concentration in soils have been reported by other workers[54,55 ,56]. The strong association between soil Cu and the organic matter is also in agreement with the general finding that Cu forms stable complexes with soil humus[57]. The amounts of non-residual fractions (F1, F2 and F3) represents the amounts of active heavy metals while those of the residual fractions may be considered to be the stable form and thus not available to plants for a reasonable period[58]. In this research, the average percentage proportions of Cu and Zn in the non-residual fractions of the soil were 48.77% and 74.78% respectively suggesting that Zn was more mobile and bioavailable. Studies have shown that the residual fraction is a relatively stable and weakly available fraction and that its proportion reflects the native metal concentration in soil and have little or no environmental significance[59,60]. Although this latter assumption might be questionable, studies have shown that the removal of metals from the residual fraction requires the use of aggressive extracting solutions[61,62].
3.3. Mobility and Mobility Factor (MF) and Recovery
- The form of the element is very important in assessing its effect as a soil contaminantbased onits uptake and related phytotoxic effects[63,64 ,65]. The average Mobility Factors(MF), calculated as a percentage of the concentration of metal in the exchangeable fraction and its pseudo total concentration (sum of metal concentration levels in all the four fractions) were found to be about 24% for Zn and 10% for Cu as shown in table 2. This infers a moderately high mobility and hence bioavailability of the metals[66,67 ,68]. Studies have shown that heavy metals are potentially available for plant uptake, if the Mobility Factor is above 10%[69,70].These high mobility factors are further indication that the metals originated from anthropogenic sources[4,68,71 ,72].
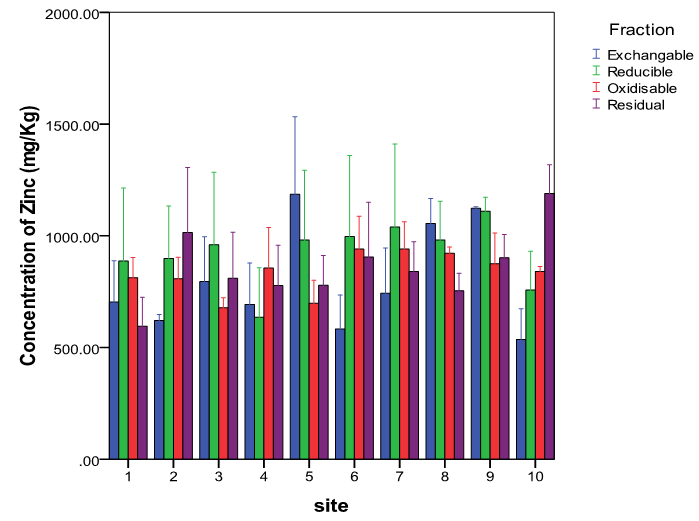 | Figure 2. Variations in the concentrations of Zn in the four fractions at the ten sites |
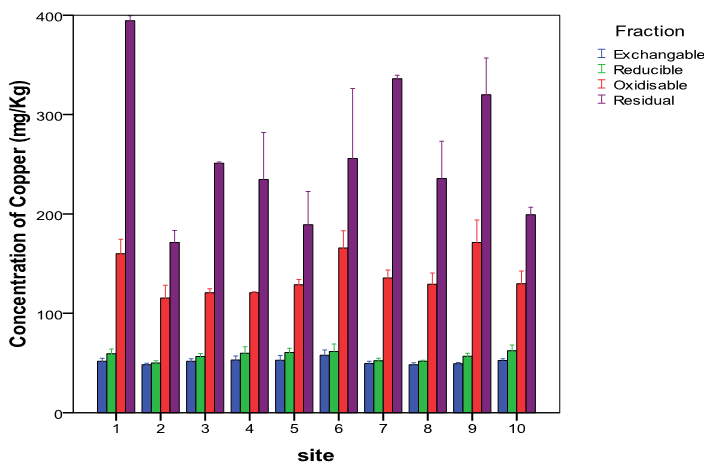 | Figure 3. Variations in the concentrations of Cu in the four fractions at the ten sites |
- It is worth noting that the mobility factors, MF of the metals varied significantly at the ten sites as can be inferred from Figure 2 for Zn and Figure 3 for Cu. For Zn it ranged from 17.02 % to 34.77% while for Cu from 7.82% to 12.30%. These variations are further evidence that their sources are from human interferences with nature and/or induced by various sources of contaminations at the sites[45].Thepseudo total concentration of the sequential extraction calculated as a sum of four fractions was found to be 505.05±5.00mg/kg for Cu for Cr and 3431.32±57.44mg/kg for Zn.The recovery total concentration of the sequential extraction was calculated as a percentage of calculated pseudo total concentration divided by the directly measured total concentration and found to range from96.14% to 102.89% as shown in Table 3[73]. This recovery range shows that the results obtained from single digestion with aqua regia were in good agreement with those of the sequential extraction procedure[74].
|
|
3.4. Soil Physicochemical Properties (pH and Total Organic Carbon TOC)
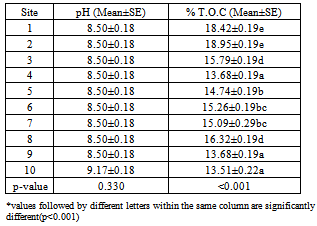
- The soil samples were found to be oily, greasy and their pH ranged from 8.5 to 9.17 as shown in Table 4. ThesepH values were higher than those reported for garage soils by other workers[75,76 ,77]. This type of alkalinityis common in anaerobic soils as a result of oxygendeficiency[78,79 ,80 ,81]. Heavy metals are mostly soluble and available at low pH[82]. The solubility of Zn and Cu was reported to be higher at pH range 4-5 than at pH 5-7. Zinc is relatively mobile at low pH, while Cu is lowly mobile[83]. The values of the Total Organic Content, T.O.C obtained varied significantly and ranged between 13.51-18.95% as shown in Table 4.
3.5. Interrelationships among Various Fractions, pH and TOC
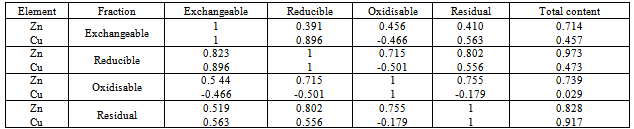
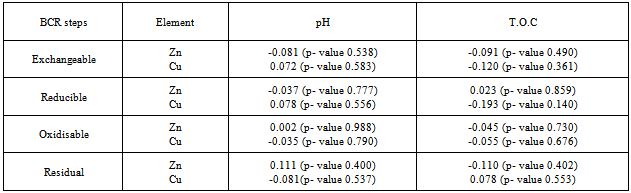
- Table 5 gives Pearson correlation values between the metal concentration levels of the various fractions with their total concentrations in the soil. A strong positive correlation was found to existbetween the concentrations of Cu in the exchangeable and those in the reducible fractions. Concentrations of Zn in the exchangeable fraction correlated positively with those in the oxidisable fractions. In addition a certain positive correlation was observed between the concentration levelsof both Zn and Cu in the exchangeablewith those found in the residual fractions. Furthermore a strong positive correlation was observed between the concentrations of Zn and Cu in the exchangeable fraction and their total concentrations in the soil. The concentration levels of Cu and Zn in the reducible fractions were also found to have a strong positive correlation with those in the exchangeable fractions. A similar observation was also made between the concentrations in the reducible and the oxidisable fractions for Zn and between the concentrations in reducible and residual fractions with the total concentrations for Zn and Cu.The significant positive correlation of concentration levels in the exchangeable fraction with the total concentration levelsof the metals implies that soils with high total concentration level will also have high amounts that are mobile.Some researchers have shown that a correlation exists between the exchangeable heavy metal concentrations and some physicochemical properties[84,85]. In the first BCR step, the concentration of Cu in the exchangeable (acid–soluble) fractionshowed a positive correlation with the pH and a negative one with the T.O.C. For the second step of the BCR method, the reducible fraction, the concentration of Cu presented a high positive correlation with the pH while that of Zn had a certain positive correlation with T.O C. For the oxidisable fraction, only Zn concentrationsshowed a certain positive correlation with pH while the concentrations of the two elements correlated negatively with T.O.C. Zn concentrations in the residual fraction correlated positively with pH while only that of Cu correlated positively with T.O.C as shown in Table 6.
|
|
3.6. Concentration of Zn and Cu in Grass and Water
- As expected from the high concentrations and mobility factors obtained, the levels in the nearby grass, water pools and run offs were high. Furtherconcentration levels of the metals down stream were slightly higherthan those found up stream. This is evidence that the metals were translocating from the garage soils to the surrounding environment. Cu was found to have higher concentration than Zn in the nearby grass as shown in Table 7 despite its lower concentration levels and mobility factors. This suggests that grass is a bioaccumulator of Cu. Since heavy metals can be transferred through food chain, there are a potential risk to ruminant animals grazing within or near automobile mechanic garages[86]. The levels of Zn and Cu in water ranged from; 26.52 to 44.63 and 1.58 to 4.45 mg/kg respectively. The concentrations of the metals in water near and within the garage were significantly high indicating considerable pollution. This means that the metals may leach into surface and ground water thus affecting their quality[87].
4. Conclusions
- These results indicate that Zn and Cu concentration levels in the top 10.0 cm of soil at the ten sampling points are well above the maximum allowed limits and have caused severe to excessive contamination capable of serious ecological and public health hazards. This implies that metal concentration levels at the study site are not from natural geology,weathering processes or deposition. The study also revealed the geochemical nature of the two metals and their probable association with different chemical forms in the soils within and around the automobile mechanic workshops. The results further showed that the metal concentration levels found in the non-residual fractions were higher than those observed in the residual fractions for Zn and that high percentages of Zn was found in a form readily available for introduction into the food chain. The relatively high mobility factors observed in the metals confirms the high lability, and biological availability of the metals in the soils studied. Thus the metals studied pose environmental risks. Consequently an environmentally friendly automobile mechanic workshop management concept that emphasizes on proper procedures of waste disposalshould be established. The poor infrastructural development common at such workshops calls for immediate action. The workshops should be properly planned and mechanics made to operate under defined code of practices. Continuous education and training thatemphasizes on the environmental implications of poor waste management and proper waste management proceduresshould be provided to them. Efficient and affordable soil remediation programs are also recommended at the sites.
References
| [1] | Alloway, B.J. (1995). Heavy metals in soils, Blackie Academic & Professional, an imprint of Chapman & Hall, London.Pp.11-37. |
| [2] | Kabata-Pendias, A., and Mukherjee, A.B. (2007).Trace Elements from Soil to Human: Springer-Verlag, Berlin. |
| [3] | Chen T.B., Wong, J.W.C., Zhou, H.Y., Wong, M.H. (1997). Assessment of trace metal distributionand contamination in surface soils of Hong Kong. EnvironmentalPollution;96:61-68 |
| [4] | Tack, F.M. G. and Verloo, M.G. (1995). Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. International Journal of Environmental Analytical Chemistry59: 225-238. |
| [5] | McLaren, R. G., Clucas, L. M., Taylor, M. D. and Hendry, T. (2003). Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge 1. Leaching of macronutrients.Australian Journal of Soil Research 41:571-588. |
| [6] | McLaughlin, M. J. and Smolders, E. (2001). Background zinc concentrations in soil affect the zinc sensitivity of soil microbial processes–a rationale for a metalloregion approach to risk assessment. Environmental Toxicology and Chemistry 20:2639–2643. |
| [7] | Speir, T. W., Van-Schaik, A. P., Percival, H. J., Close, M. E. and Pang, L. P. (2003). Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land. Water, Air and Soil Pollution 150:319-358. |
| [8] | Zarcinas, B. A., Ishak, C. F., McLaughlin, M. J., Cozens, G. (2004). Heavy metals in soils and crops in Southeast Asia. 1. Peninsular Malaysia. Environmental Geochemistry and Health 26:343-357. |
| [9] | Fernandes, J. C. and Henriques, F. S. (1991).Biochemical, physiological and structural effects of excess copper in plants.The Botanical Review57: 246-273. |
| [10] | Adriano, D. C. (1986).Trace elements in the terrestrial environment. Springer-Verlag New York 105–123, 533. |
| [11] | Wang, C.X., Mo, Z., Wang, H., Wang, Z. J. and Cao, Z. H. (2003).The transport, time-dependent distribution of heavy metals in paddy crops.Chemosphere 50: 717-723. |
| [12] | Lu, A., S. Zhang and X-Q.Shan, 2005. Time effects on the fractionation of heavy metals in soils. Geoderma, 125: 225-234. |
| [13] | Sharma, R. K., Agrawal, M. and Marshall, F. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India.Ecotoxicology and Environmental Safety 66: 258 – 266. |
| [14] | Gupta, S.K., Vollmer, M.K. and Krebs, R. (1996). The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management. Science of the Total Environment.178, 11-20. |
| [15] | Xian, X. (1989).Effect of chemical forms of cadmium, zinc, and lead in polluted soils on their uptake by cabbage plants.Plant Soil 113:257-264. |
| [16] | Lonati, G., Giugliano, M. and Cernuschi, S. (2006). The role of traffic emissions from weekends and weekday fine PM data in Milan.Atmospheric Environment 40: 5998-6011. |
| [17] | Pecheyran, C., Lalere, B. and Donald, O. F. X (2000).Volatile metal and metalloid species (Pb, Hg, Se in a European urban atmosphere (Bordeaux, France).Environmental Science and Technology 34:27-32. |
| [18] | Onianwa, P. C., Jaiyeola, O. M. and Egekenze, R. N., (2001). Heavy metal contamination of top soil in the vicinities of auto-repair workshops, gas stations and motor parks in a Nigerian city Toxicology and Environmental Chemistry 84: 33-39. |
| [19] | Ipeaiyeda, A. R. and Dawodu, M. (2008). Heavy metals contamination of topsoil and dispersion in the vicinities of reclaimed auto-repair workshops in Iwo Nigeria.Bulletin of Chemical Society Ethiopia22:339-348. |
| [20] | Iwegbue, C. M. A. (2007). Metal fractionation in soil profiles at automobile mechanic waste dumps. Waste Management Research 25: 585-593. |
| [21] | Soon, Y. K. and Bates, T. E. (1982).Chemical pools of cadmium, nickel, and zinc in polluted soils and some preliminary indications of their availability to plants.Journal of Soil Science 33: 477-488. |
| [22] | Akpoveta, O. V., Osakwe, S. A., Okoh, B. E. and Otuya, B. O. (2010). Physicochemical characteristics and levels of some heavy metals in soils around metal scrap dumps in some parts of Delta State, Nigeria. Journal of Applied Science and Environment 4: 57-60. |
| [23] | Iwegbue, C. M. A., Nwajei, G. E., Eguavon, O. and Ogala, J.E. (2009). Chemical fractionation of some heavy metals in soil profiles in vicinity of scrap dumps in Warri, Nigeria. Chemical Speciation and Bioavailability 21: 99-110. |
| [24] | Ahumada, I., Escudero, P., Adriana, C. M., Castillo, G., Ascra, L. and Fuentes, E. (2004).Use of sequential extraction to assess the influence of sewage sludge amendment on metal mobility in Chilean soils.Journal of Environmental Monitoring 6: 327-334. |
| [25] | Albores, A.F., Perez-cid, B., Gomes, E. F. and Lopez, E.F. (2000). Comparison between sequential extractionprocedures and single extraction procedures and for metal partitioning in sewage sludge samples Analyst 125:1353–1357. |
| [26] | Norvell W.A. (1984) Comparison of chelating agents for metals in diverse soil materials, Soil Science Society of America Journal. 48: 1285 – 1292 |
| [27] | Ahumada, I., Escudero, P., Adriana, C. M., Castillo, G., Ascra, L. and Fuentes, E. (2004).Use of sequential extraction to assess the influence of sewage sludge amendment on metal mobility in Chilean soils.Journal of Environmental Monitoring 6: 327-334. |
| [28] | Khairiah, J., Ding-Woei, Y., Habibah, J., Ahmad-Mahir, A., Aminah, A. and Ismail, B. S. (2009).Concentration of heavy metals in guava plant parts and soil in the Sungai Wangi Plantation, Perak, Malaysia.International Journal of Agricultural Research. 4: 310-316. |
| [29] | Ure, A. M. and Davidson, C. M. (2002).Chemical Speciation in the Environment. Blackwell, Oxford. |
| [30] | Rauret, G. (1998). Extraction procedures for the determination of heavy metals in contaminated soil and sediment.Talanta 46: 449-455. |
| [31] | Amanda, J. Z and Weindorf, D. C. (2010). Heavy metal and trace metal analysis by sequential extraction: A review of procedures. International Journal of Analytical Chemistry 1-7. |
| [32] | Song, Q. J. and Greenway, G. M. (2004).A study of the elemental leachability of and retention capability of composts.Journal of Environmental Monitoring 6:31-37. |
| [33] | Central Bureau of Statistics (CBS), (2010). Kenya population census 1999, Ministry of Planning and National Development, Nairobi Kenya. |
| [34] | United Nations Environmental Protection Programme (UNEP), Nairobi. (2004). Sustainable consumption and production activities in Africa: Regional status report, 2002-2004. Nairobi Kenya. |
| [35] | Ure, A. M., Davidson, C. M., Thomas, R. P. (1995). Single and sequential extraction schemes for trace metal speciation in soil and sediment. In: Quality assurance for environmental analysis. Elsevier Science B.V20: 505–523. |
| [36] | Fagbote, E. O. and Olanipekun, E. O. (2010). Evaluation of the status of heavy metal pollution of sediment of Agbabu bitumen deposit area, Nigeria. Eur. J. Sci. Res. 41(3): 373-382 |
| [37] | Charles, M. J. and Simmons, M. S. (1986). Methods for the determination of carbon in soils and sediments: A review. Analyst 111:385-390. |
| [38] | Broadbent, F. E. (1953).The soil organic fraction.Advances in Agronomy5: 153-183. |
| [39] | [39]Nelson, D. W. and Sommers, L. E. (1996).Total carbon, organic carbon, and organic matter. In: Methods of Soil Analysis, Part 2, 2nd ed. Page, A. L., ed. American Society of Agronomy Inc. Madison, WI. 9: 961-1010. |
| [40] | Broadbent, F. E. (1953).The soil organic fraction. Advances in Agronomy5: 153-183. |
| [41] | Soil Survey Laboratory Methods Manual. (1992). Soil Survey Investigations Report No. 42. U.S. Department of Agriculture, Washington, DC. |
| [42] | Kholoud, M., Mohammed, A. and Yahya, A. (2009) Spatial Distribution and Environmental Implications of Lead and Zinc in Urban Soils and Street Dusts Samples in Al-HashimeyehMunicipalityJordan Journal of Mechanical and Industrial Engineering Jordan Journal of Mechanical and Industrial Engineering2:141 – 150. |
| [43] | Council Directive 86/278/EEC (1986): On the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. European Commission Official Journal L 181. |
| [44] | Ayodele, J. T. and Oluyomi, C. D. (2011).Grass contamination by trace metals from road traffic. Journal of Environmental Chemistry and Ecotoxicology 3:60-67. |
| [45] | Kabata-Pendias, A. (1995). Agricultural problems related to excessive trace metal contents of soil. In: Heavy metals (problems and solutions). Salomons, W., Förstnerand, U. and Mader, P. Eds. Springer Verlag, Berlin. 3-18. |
| [46] | Lindsay, W. L. (1979). Chemical equilibria in soils. John Wiley and Sons, New York. |
| [47] | Kholoud, M., Mohammed, A. and Yahya, A. (2009) Spatial Distribution and Environmental Implications of Lead and Zinc in Urban Soils and Street Dusts Samples in Al-Hashimeyeh MunicipalityJordan Journal of Mechanical and Industrial Engineering Jordan Journal of Mechanical and Industrial Engineering2:141 – 150. |
| [48] | Surthland, R. A., Tolosa, C. A., Tack, F. M. G. and Verloo, M. G., (2000).Characterization of selected element concentration and enrichment ratios in background and anthropogenically impacted roadside areas.Archives of Environmental Contamination and Toxicology 38: 428–438. |
| [49] | Kabata-Pendias, A. (1995). Agricultural problems related to excessive trace metal contents of soil. In: Heavy metals (problems and solutions). Salomons, W., Förstnerand, U. and Mader, P. Eds. Springer Verlag, Berlin. 3-18. |
| [50] | Kuo, S., Heilman, P. E. and Baker, A.S. (1983). Distribution and forms of copper, zinc, cadmium, iron and manganese in soils near a copper smelter. Soil Science 135:101-109. |
| [51] | Ramos. L., Hernandez, L. M. and Gonzales, M. J. (1994). Sequential fractionation of copper, lead, cadmium and zinc in soil from or near Donana national park. Journal of Environmental Quality23: 50–57. |
| [52] | Chao, T. T. (1984). Use of partial dissolution techniques in exploration geochemistry: Journal of GeochemicalExploration 20:101-135. |
| [53] | Kashem, M. A., Singh, B. R., Kondo, T., Imamul-Huq, S. M. and Kawai, S. (2007). Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non-contaminated soils.International Journal of Environmental Science and Technology 4: 169-176. |
| [54] | Shuman, L. M. (1985).Fractionation method for soil micro elements.Soil Science Society of America Journal 140:11-22. |
| [55] | Shuman, L. M. (1979). Zinc, manganese and copper in soil fraction. Soil Science Society of America Journal 127: 10-17. |
| [56] | Harrison, R. M. (1981).Chemical association of lead, Cd, Cu, and Zn in street dusts and roadside soils.Environment Science and Technology 15: 1378-1383. |
| [57] | Stumm, W., and Morgan, J. J. (1981). Aquatic chemistry: An introduction emphasizing chemical equilibria in natural waters. 2nd ed. John Wiley & Sons, New York. |
| [58] | Sposito, G. (2008).The chemistry of soils 2nd edition: Oxford University Press. Madison Arenne, New York. |
| [59] | Kersten, M. and Förstner, U. (1986).Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments.Water Science and Technology 18: 121-130. |
| [60] | Kabala, C. and Singh, B. R. (2001). Fractionation and mobility of copper, lead, and zinc in soil profile in the vicinity of a copper smelter. Journal of Environmental Quality 30:485-495. |
| [61] | Chao, T. T. (1984). Use of partial dissolution techniques in exploration geochemistry: Journal of GeochemicalExploration 20:101-135. |
| [62] | Kashem, M. A., Singh, B. R., Kondo, T., Imamul-Huq, S. M. and Kawai, S. (2007). Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non-contaminated soils.International Journal ofEnvironmental Science and Technology 4: 169-176. |
| [63] | Olajire, A. A., Ayodele, E. T., Oyediran, G. O. and Oluyemi, E. A. (2003).Levels and speciation of heavy metals in soils of industrial southern Nigeria.Environmental Monitoring and Assessment 85:135-155. |
| [64] | Sauve, S. (2001).Speciation of metals in soils. Bioavailability of metals in terrestrial ecosystems: Importance of partitioning for bioavailability to invertebrates. Microbes and Plants 7-38. |
| [65] | Sauve, S. (2003).The role of chemical speciation in bioavailability.Bioavailability, toxicity and risk.Relationships in Ecosystems 59-82. |
| [66] | Kersten, M. and Förstner, U. (1986).Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments.Water Science and Technology 18: 121-130. |
| [67] | Kabala, C. and Singh, B. R. (2001). Fractionation and mobility of copper, lead, and zinc in soil profile in the vicinity of a copper smelter. Journal of Environmental Quality 30:485-495. |
| [68] | Ma, L. Q. and Rao, G. N. (1997).Chemical fractionation of cadmium, copper, nickel and zinc in contaminated soils.Journal of Environmental Quality 26: 259-264. |
| [69] | Kersten, M. and Förstner, U. (1986).Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments.Water Science and Technology 18: 121-130. |
| [70] | Kabala, C. and Singh, B. R. (2001). Fractionation and mobility of copper, lead, and zinc in soil profile in the vicinity of a copper smelter. Journal of Environmental Quality 30:485-495. |
| [71] | Council Directive 86/278/EEC (1986): On the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. European Commission Official Journal 181. |
| [72] | Chao, T. T. (1984). Use of partial dissolution techniques in exploration geochemistry: Journal of GeochemicalExploration 20:101-135. |
| [73] | Gupta, S. K and Chen, K. Y (1975). Partitioning of trace metal in selective chemical fractions of near shore sediments. Environmental Letters 10:129-158. |
| [74] | Madrid, L., Díaz-Barrientos, E. Madrid, F. (2002). Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere 49: 1301–1308. |
| [75] | Tukura, B W., Kagbu, J A. and Gimba, C. E. (2007). Effects of pH and total organic carbon on the distribution of trace metals in Kubanni dam sediments, Zaria, Nigeria. Science World Journal 2:1-6. |
| [76] | Iwegbue, C. M. A., Nwajei, G. E., Eguavon, O. and Ogala, J.E. (2009). Chemical fractionation of some heavy metals in soil profiles in vicinity of scrap dumps in Warri, Nigeria. Chemical Speciation and Bioavailability 21: 99-110. |
| [77] | Oviasogie, P. O. and Ofomaja, A. (2007). Available Mn, Zn, Fe, Pb and physicochemical changes associated with soil receiving cassava mull effluent. Journal of Chemical Society of Nigeria 32:69-73. |
| [78] | Isirimah, N. O. (1987). An inventory of some chemical properties of selected surface soils of River state of Nigeria: In proceeding of the 15th annual conference of soil science association of Nigeria, Kaduna. 217-233. |
| [79] | Odu, C. T. I., Esurosu, O. F., Nwaboshi, I. C. and Ogunwale, J.A. (1985).Environmental study (Soil and Vegetation) of Nigeria Agip oil company operation area. A report submitted to Nigeria Agip Oil Company Limited, Lagos, Nigeria. 102-107. |
| [80] | Sauvé, S., Hendershot, W. and Allen, H. E. (2000). Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environmental Science and Technology34: 1125-1131. |
| [81] | Osakwe, S. A. and Egharevba, F. (2008).Sequential Fractionation of cadmium, copper, lead and chromium, in soil around, Municipal Solid Waste Dumps in Agbor, Nigeria. J. Chem. Soc. Nig. 33: 139 – 147 |
| [82] | Munoz-Melendez, G., Korre, A. and Parry, S. J. (2000). Influence of soil pH on the fractionation of Cr, Cu and Zn in solid phases from a landfill site. Environmental Pollution 110:497–504. |
| [83] | Kabata-Pendias, A. and Pendias, H. (2001). Trace elements in soils and plants. 3rd ed. Boca Raton: CRC Press USA. |
| [84] | Zhang, T., Shan, X. and Fuliang, L. (1998).Comparison of two sequential extraction procedures for speciation analysis of metals in soils and plant availability.Communication in Soil Science and Plant Analysis 29: 1023-1034. |
| [85] | Zhang, H. and Young, S. D. (2005).Characterizing the availability of metals in contaminated soils.The soil solution Soil Use and Management 21:459-467. |
| [86] | Ayodele, J. T. and Oluyomi, C. D. (2011).Grass contamination by trace metals from road traffic. Journal of Environmental Chemistry and Ecotoxicology 3:60-67. |
| [87] | United Nations Environmental Protection Programme (UNEP), Nairobi. (2004). Sustainable consumption and production activities in Africa: Regional status report, 2002-2004. Nairobi Kenya. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML