-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2013; 3(4): 91-99
doi:10.5923/j.re.20130304.06
Environmental Assessment of Climate Smart Agricultural Interventions in Smallholder Crop-livestock Production Systems
Swidiq Mugerwa1, Emmanuel Zziwa2, 3, Jolly Kabirizi1, Jean Ndikumana3
1National Livestock Resources Research Institute, P.O. Box 96, Tororo, Uganda
2Makerere University Kampala, Uganda, P.O. Box 7062, Kampala, Uganda
3Association for Strengthening Agricultural Research in Eastern and Central Africa, P.O. Box 765, Entebbe, Uganda
Correspondence to: Swidiq Mugerwa, National Livestock Resources Research Institute, P.O. Box 96, Tororo, Uganda.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The study evaluated the environmental effects of various climate smart agricultural interventions (CSAI) in the smallholder production systems of Uganda. Soil fertility management interventions improved soil pH, soil nitrogen, organic matter, earthworm density and microbial biomass by 8, 55, 94, 130 and 44.2% respectively. Installation of roof-top water harvesting tanks ensured availability of good quality water whose total coliform concentration was 529, 967 and 1400% lower than in spring wells, ponds and run-off water harvesting tanks respectively. Also, fecal coliform concentration in roof-top water harvesting tanks was 17 and 28 times lower than in spring wells and ponds respectively. The methane-milk ratio for feed package A was 54 and 97% higher than the ratios for feed packages B and C. The results of the study implied that in the face of climate change and variability, CSAI have the potential to sustain high productivity of crop livestock through enhancing soil fertility, water availability and feed utilisation of animals. The study called for further research to establish appropriate manure application rates for different crops and manure types as well as to improve the quality of water collected in run-off water harvesting tanks.
Keywords: Water, Soil, Air, Climate Change, Smart Agriculture, Uganda
Cite this paper: Swidiq Mugerwa, Emmanuel Zziwa, Jolly Kabirizi, Jean Ndikumana, Environmental Assessment of Climate Smart Agricultural Interventions in Smallholder Crop-livestock Production Systems, Resources and Environment, Vol. 3 No. 4, 2013, pp. 91-99. doi: 10.5923/j.re.20130304.06.
Article Outline
1. Introduction
- In Africa the demand for agricultural products such as maize and pulses is set to increase at 3-4% per year while livestock production has to triple by 2030 to meet the increasing demand from the growing population. However, the escalating demand for agricultural products is taking place at a time when climatic variability and change are significantly undermining agricultural productivity. Moreover, a limited number of smallholder farmers have embraced climate resilient farming practices to increase food production. As such crop yields have continued to decline threating food security of the increasing population. For example, between 2006 and 2009 crop and livestock losses due to extreme weather events especially floods in Rwanda were estimated at USD 4-22 million per annum for only 2 districts[1]. In Kenya, an estimated 2.2 million people in pastoral areas, the southeastern and coastal marginal agricultural areas faced food insecurity in 2012 due to prolonged drought whereas in the Karamoja sub-region of Uganda, severe and widespread droughts affected the sub-region during 2006 and 2008[2], with devastating impacts on crop yields (>70% decline in yields), livestock populations, rangeland health and eventually food security. Such devastating and yet inevitable effects of climate change and variability on the productivity, profitability and sustainability of agricultural production systems heightened the impetus to bolster the resilience and adaptive capacity of agricultural systems particularly the stallholder crop - livestock farming systems that employ over 70% of the human population in Eastern and Central African (ECA) region[3,4]. As such, the Association for Strengthening Agricultural Research in Eastern and Central Africa (ASARECA) has in recent times committed funds to research and development projects that seek to promote climate smart agricultural interventions in smallholder production systems to strengthen households’ resilience to the adverse effects of climate change and variability. One of such projects is“Harnessing crop-livestock integration to build resilience of smallholder crop-livestock production systems in ECA region”, implemented in Burundi, Kenya, Rwanda, Tanzania and Uganda. The project promotes various climate smart interventions including soil fertility management and nutrient recycling interventions, water harvesting innovations for agricultural and domestic uses, and appropriate livestock dietary packages among others. None the less, several agricultural practices have been noted to cause environmental pollution heightening the impetus for continued assessment of the impacts of agricultural interventions on the environment[5]. The study thus presents findings on the effect of project interventions on selected environmental parameters particularly on soil, air and water quality in smallholder agricultural production systems of Uganda.
2. Materials and Methods
2.1. Study Sites
- The study was conducted in Kitenga village, Kalagala Parish, Mukungwe Sub-county, Masaka District, Uganda (Figure 1). Masaka District is located 0° 15° and 0° 43° south of the equator and 310 and 320 East, with average altitude of 115m above sea level. The district has a total area of 6413.3 km2 with the total land area being 3214 km2. The soils are generally ferralitic clay loams. It has a tropical climate with bimodal rainfall pattern characterized by two rainy seasons with dry spells occurring between January to March and July to August. The mean daily temperature ranges between 10℃ - 30℃. The major economic activity is agriculture (smallholder crop - livestock production systems). Constraints to agriculture production are prolonged droughts that lead to crop failure and increased feed scarcity.
2.2. Description of Project Interventions
- Soil fertility management and nutrient recycling interventions included utilization of animal manures (both poultry and cattle manure) from zero grazing and poultry units for crop and forage production. Particularly, the project promoted the cultivation of leafy vegetables using cattle manure (VCM) or poultry manure (VPM). The project also introduced and promoted the cultivation of drought tolerant forages particularly Brachiaria mulato (1) to mitigate drought-induced forage scarcity. Emphasis was put on the production of Brachiaria mulato (1) using cattle manure obtained from animals (BCM). Also, the project promoted the integration of forage legumes into Napier grass based fodder production systems (NL) as a means of enhancing the nutritive value of the forage offered to animals as well as improving the nitrogen composition of soils through nitrogen fixation. As such, the soil fertility and nutrient recycling interventions evaluated in the current study include VCM, VPM, BCM and NL.Before project interventions were implemented on beneficiary farmers’ farms, soil samples were collected from the farmers’ cropland to act as a benchmark against which changes induced by project intentions would be assessed. This benchmark is hereafter denoted with letter “C”. In terms of water harvesting interventions, the project installed roof-top (RT) and run-off (ROT) water harvesting facilities. The quality of water collected in the two water harvesting structures was compared to the quality of water in community water sources where beneficiary farmers used to collect water before project interventions. The community water sources included spring wells (SW) and ponds (P) dug out in swamps. Also, the quality of water in the installed facilities was compared with piped tap water (TW) which is provided by the National Water and Sewerage Corporation of Uganda. In terms of livestock nutrition, the project introduced forage legumes (such as Lablabpurpureus and Centrosemapubescens) into the feeds and feeding systems of smallholder crop-livestock farmers and the quantity of methane emitted by animals feeding on feed packages containing forage legumes was simulated and compared to the values for feed packages deficient in forage legumes.
2.3. Data Collection and Analysis
- Five soil samples were taken to a depth of 0-15cm with an auger from each plot subjected to VCM, VPM, BCM and NL interventions one and half years after manure application. The samples were thoroughly mixed and bulked to form one composite sample per plot per household. The samples were analyzed for Nitrogen (N), Organic matter (OM), Magnesium (Mg), Sodium (Na), Calcium (Ca), available Phosphorus (P), Potassium (K) and soil pH according to methods described by[6]. The Standard Tropical Soil Biology and Fertility (TSBF) method[7] that consists of hand-sorting macro-invertebrates from soil monoliths measuring 25 cm width, 25 cm length and 30 cm depth was used to sample and quantify the density of earthworms. The soil microbial biomass was estimated according to methods described by[8]. Water samples were collected from roof-top and runoff water harvesting facilities, community water sources (spring well and ponds) and piped water system (water taps) at the end of the second rainy season in 2012. The samples were delivered to the laboratory in ice boxes where they were frozen until analysis was made within 36 hours. A spectrophotometer (HACH, DR/4000) was used to measure nitrites, nitrates, ammonia, total nitrogen, total phosphorus, total dissolved solids and Turbidity. Total suspended solids (TSS) were determined by filtering and weighing oven dried sediments collected on pre-washed Whatman GF/F Glass filters. Soil pH was measured using WTH pH meter LF 90. Total and fecal coliform bacteria levels were determined by the membrane lauryl sulfate broth method after incubation at 44℃ for 18 to 24 hours. The LIFESIM model developed by International Potato Center (CIP), 2006[9] was used to simulate methane emissions from the different dietary packages utilised by farmers. The minimum number of replications per intervention was four and the mean value for all the replications for each parameter per intervention was used to generate graphs in XLSTAT[10]. Each beneficiary farmer was therefore regarded as a replicate for a particular intervention.
 | Figure 1. Map of Uganda showing the location of Mukungwe sub-county and Kalagala Parish |
3. Results
3.1. Soil Quality
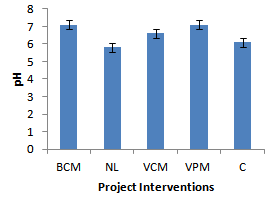 | Figure 2. Effect of project interventions on soil pH |
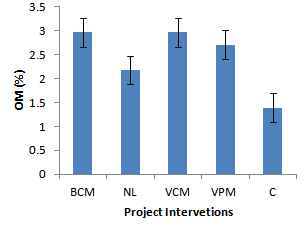 | Figure 3. Effect of project interventions on OM |
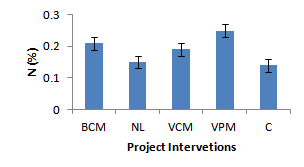 | Figure 4. Effect of project interventions on soil N2 |
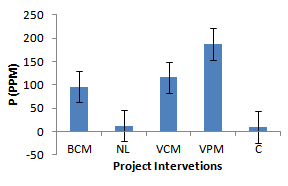 | Figure 5. Effect of project interventions on soil P |
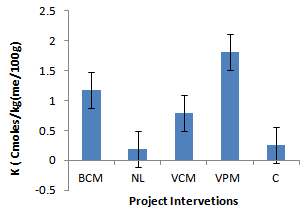 | Figure 6. Effect of project interventions on K ions |
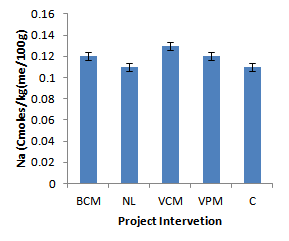 | Figure 7. Effect of project interventions on Na ions |
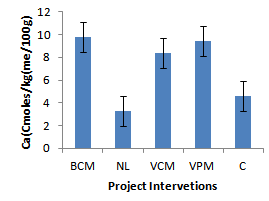 | Figure 8. Effect of project interventions on Ca ions |
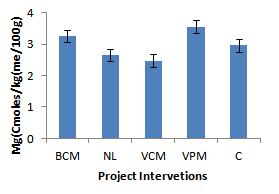 | Figure 9. Effect of project interventions on Mg ions |
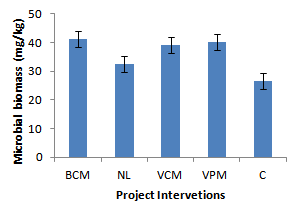 | Figure 10. Effects on soil microbial biomass |
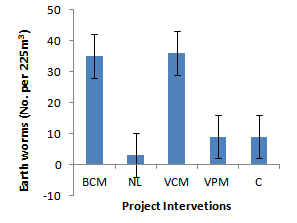 | Figure 11. Effects of project interventions on earthworm density |
3.2. Water Quality
- Installation of roof-top water harvesting tanks by the project ensured availability of water with low microbial contamination to smallholder crop livestock farmers. The concentration of total coliforms in roof-top water harvesting tanks was 529 and 967% lower than the concentration in spring wells and ponds respectively (Figure. 12). Further, the concentration of total coliforms in roof-top water harvesting tanks was 1400% lower than the concentration in run-off water harvesting tanks. The same trend was noted for fecal coliform concentration were the value in roof-top water harvesting tanks was 17 and 28 times lower than the values in spring wells and ponds respectively (Figure. 13). Also, the concentration of fecal coliforms in roof-top water harvesting tanks was 18 times lower than the concentration in run-off water harvesting tanks. No fecal coliform contamination was found in piped tap water and water from roof-top water harvesting tanks. The lowest value of total suspended solids was obtained in roof-top water harvesting tank and was 75, 325, 337 and 338% lower than the values from tap water, ponds, spring wells and run-off water harvesting tanks respectively. The turbidity of water from roof-top water harvesting tanks was 33, 367 and 567% lower than the values for run-off water tanks; pond and spring wells respectively (Figure 15). The concentration of NH4+(Figure 20), NO2-(Figure 16), NO3-(Figure 17), total P (Figure 19) and total N (Figure 18) were all below the critical values above which water is considered unsafe for human consumption. Also, the pH of water from all water sources ranged between 6.5 and 6.8 and was within the recommended range of 6-8 (Figure 21).
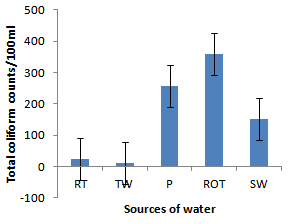 | Figure 12. Total coliforms in water sources |
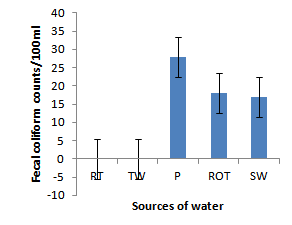 | Figure 13. Fecal coliforms in water sources |
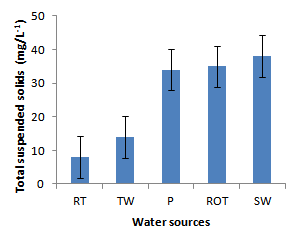 | Figure 14. Total suspended solids in water sources |
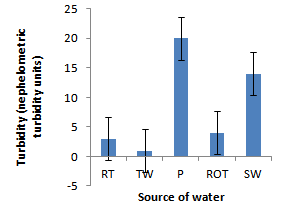 | Figure 15. Turbidity of water in water sources |
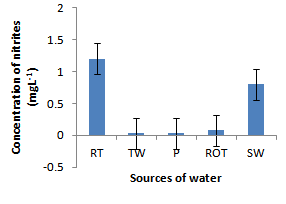 | Figure 16. Nitrites in water sources |
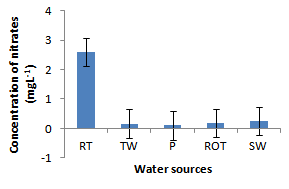 | Figure 17. Nitrates in water sources |
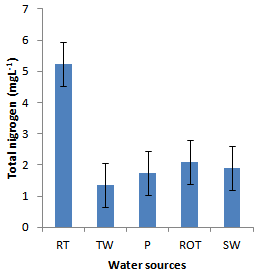 | Figure 18. Total nitrogen in water sources |
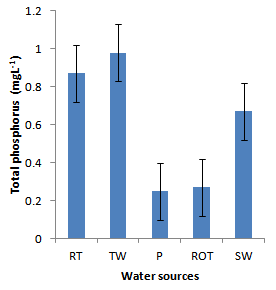 | Figure 19. Total phosphorus in water sources |
|
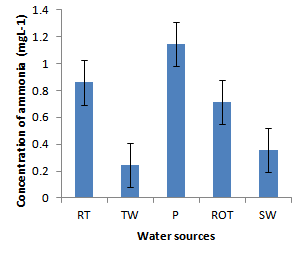 | Figure 20. Ammonia concentration in water sources |
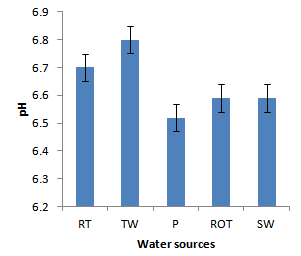 | Figure 21. pH for different water sources |
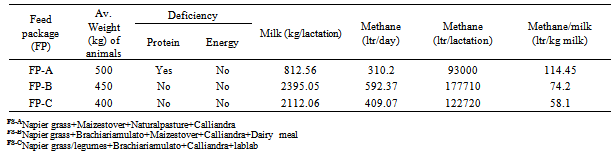
3.3. Air Quality: Simulated Methane Emissions from Dairy Cattle
- Simulation of rumen methane production indicated that dietary packages deficient in protein resulted into lower methane emissions but the same packages led to higher methane to milk ratios (Table 1). The methane to milk ratio for feed package A, the feed package mostly utilized by farmers to feed livestock before project interventions was 54 and 97% higher than the ratios for feed packages B and C respectively, promoted by the project.
4. Discussion
4.1. Soil Quality
- Neutralization of soil pH was attributed to the reduction in concentration of acid forming ions in the soil solution. When animal manures are added to soils, the manures get mineralized leading to release of Ca2+ ions into the soil solution which quickly get hydrolysed to form Calcium hydroxide. The Calcium hydroxides then react with soluble aluminum ions in the soil solution to give insoluble Al(OH)3. The hydroxide of the Aluminium then reacts with hydrogen ions to form water and reducing the concentration of acid forming ions[11]. These results are in agreement with findings of Whalen et al., 2000; Naramabuye et al., 2007 and Mugerwa et al., 2000;[12],[13] and[3] who also reported the liming effect of animal manures on soil pH. The improvement in the soil chemical properties was attributed to the mineralization of cations from animal manures while the difference in the effect on soil properties among the different project interventions was partly due to variation in manure quantities and quality applied by the different farmers. Previous studies also recognized the role of animal manures in improving soil nitrogen[14]; soil organic matter and Phosphorus[15]. Increase in the activity and population of soil organisms was attributed to the increased availability of sources of nourishment for soil organisms[11].
4.2. Water Quality
- As surface runoff moves down slope, it collects various materials including animal manure and sediments which are then deposited in downstream water reservoirs. This greatly increases total coliforms, fecal coliforms, TDS, TSS, and Turbidity as compared to reservoirs that do not receive surface run-off. As such the total coliforms, fecal coliforms, TDS, TSS, and Turbidity of water in spring well, ponds and run-off water harvesting tanks were higher than the values for roof-top water harvesting tanks and piped tap water which are not recipients of surface runoff. Although the major source of water in springs is not surface runoff, spring wells are usually not protected from runoff and hence the clean water from springs is often contaminated by surface runoff. These results are consistent with findings of Zziwa et al, 2012 and Gadgil, et al., 1998[16, 17] who reported that contamination of water was high in reservoirs receiving surface runoff. The most alarming finding of this study is that the water formally accessed by farmers before project interventions was not safe for human consumption and for calves that are sensitive to lower levels of fecal coliforms. The maximum acceptable level of fecal coliforms in water to be regarded as safe for human consumption is actually zero (0 counts/100ml) while that of total coliforms ranges between 1 and 100 counts/100 ml. This implies that the water in community water reservoirs and runoff water harvesting facilities is not safe for human consumption. This however doesn’t mean that runoff water harvesting tanks are not beneficial in cushioning households against water scarcity. In fact, the tanks improve the amount of water available to households but the water is not safe for human consumption but can be used for other purposes such as irrigation, cleaning and even watering mature animals that are not too sensitive to high concentrations of total and fecal coliforms. Presence of fecal coliforms in water indicates that water is contaminated by pathogens of fecal origin e.g. E. coli and Cryptosporidium. These pathogens are closely associated with occurrence of waterborne pathogenic diseases in humans including ear infections, dysentery, typhoid fever, viral and bacterial gastroenteritis among other diseases[17].
4.3. Air Quality: Simulated Methane Emissions from Dairy Cattle
- The farmers’ feed package (FP-A) that was deficient in protein was associated with high methane to milk ratio compared to feed packages “C” and “D” that contained adequate protein. This relationship was also demonstrated by[18] who noted that supplementation of lactating dairy cows with protein concentrates resulted in a reduction in CH4 emissions of up to 3.5 g of CH4/kg of dry matter intake. As the protein concentration of feed stuffs is increased, both milk production and methane emissions increase because feed stuffs that provide adequate requirements for improved animal performance and production also modify the rumen environment, providing the required nutrients for increased multiplication of rumen microbes including methanogenic bacteria, hence increasing methane emissions. However, increases in beneficial rumen microbes increase rates of feed passage and efficient utilization resulting into more beneficial outputs of producing more milk per unit of methane emitted. This is also consistent with findings of Birkelo, et al., 1986 and Mugerwa, et al., 2012[19] and[20] who noted that protein supplementation of low-quality diets increases methane production but decreases methane emissions per unit of product (maintenance, lactation, or growth). Eliminating protein deficiency through integration of forage legumes into feed packages therefore provides an enormous opportunity for methane mitigation through lowering the volume produced per kilogram of milk produced.
4.4. Implications of Study Findings on Productivity of Smallholder Crop-livestock Production Systems
- Climate smart agriculture can be defined as that, which sustainably increases productivity, resilience, reduces or removes Greenhouse Gases (GHGs), and enhances achievement of national food security and development goals. In other words, Climate Smart Agriculture strategies/innovations are those that achieve so called “triple wins” of adaptation, mitigation and development. Transformation of agricultural production to climate smart agricultural production systems requires improvement in the overall efficiency, resilience, adaptive capacity and mitigation potential of the current production systems. This can be achieved by improving various components of current production systems including soil and nutrient management, water harvesting and use, and reduction in emission of GHGs. Soil fertility is a key determinant of crop/forage yields in low input smallholder crop livestock production systems. It also influences the susceptibility of crops/fodder to external shocks such pests and diseases as well as water scarcity. Although not quantified in the current study, the improvement of chemical and biological properties through nutrient management and recycling interventions is associated with improved crop/forage/fodder yield, increased resilience to external shocks and eventually improved efficiency and productivity of agricultural systems. Also, reduction in the amount of methane emitted per litre of milk produced is indicative of improved feed efficiency and animal performance while mitigating emissions of GHGs to the atmosphere. Water harvesting on the other hand improved the amount of water available for domestic and agricultural uses to mitigate the adverse effects of water scarcity attributed to climate variability. As such, implementation of climate smart agricultural interventions is fundamental in sustaining agricultural productivity in the face of climate change and variability.
5. Conclusions and Recommendations
- Amendment of crop and forage fields with animal manures significantly improved the chemical and biological properties of the soil, making it possible to enhance as well as sustain crop/forage yields in smallholder crop livestock agricultural systems. Further, the improved soil fertility would reduce the vulnerability of crops to external shocks such pests, diseases and moisture stress among others, leading to improved agricultural productivity. Also, installation of water harvesting tanks ensured availability of adequate and safe water for livestock and human consumption, mitigating the adverse effects of water scarcity attributed to climate change and variability. This would ensure crop/forage production even during periods of inadequate and erratic rains through employing simple irrigation techniques. Finally, improved feeding of lactating cows improved animal performance (feed intake and milk production) while mitigating methane emissions to the atmosphere. The study is suggestive that although the runoff water harvesting facility improved water availability, the quality of water in such tanks is not safe for human consumption. If this water is to be used, there is a need to establish simple and cost effective water treatment technologies to enhance its safety. Also, more investigations are needed to establish the appropriate manure application rates for the different crops as well as for the different manure types so as to utilize the resource more efficiently, effectively and sustainably.
ACKNOWLEDGEMENTS
- The study was funded by the Association for Strengthening Agricultural Research in Eastern and Central Africa (ASARECA) through the second implementation phase of a project titled “Harnessing crop-livestock integration to build resilience of smallholder crop-livestock production systems in ECA region”.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML