-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2012; 2(6): 271-274
doi: 10.5923/j.re.20120206.04
Phenoloxidases Produced by Endophytic Fungi Isolated from Baccharis Dracunculifolia D. C. (Asteraceae)
Sideney Becker Onofre , Paula Steilmann
Institute of Biological and Health Sciences , Department of Biological Sciences , Paranaense University (UNIPAR) , Unit of Francisco Beltrão , Francisco Beltrão , Paraná , Brazil
Correspondence to: Sideney Becker Onofre , Institute of Biological and Health Sciences , Department of Biological Sciences , Paranaense University (UNIPAR) , Unit of Francisco Beltrão , Francisco Beltrão , Paraná , Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
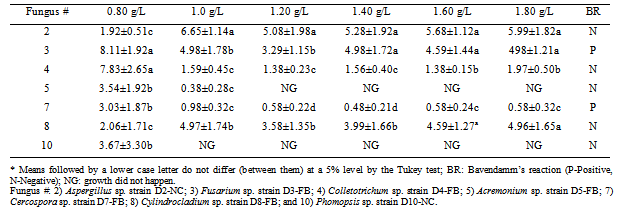
Phenolic compounds fall within the waste resulting from the biodegradation of natural and anthropogenic, are found in soil and water, but despite being widely distributed in nature are part of the main pollutants toxic waste discarded by a wide variety of industries such as textiles, petroleum refining, pulp and paper, pharmaceuticals, coating metals, wood preservatives, dyes, plastics and resins, coal conversion, and are components of many biocides. In order to remedy the impacts of these compounds, seven endophytic fungal species isolated from Baccharis dracunculifolia D. C. (Asteraceae) were studied to determine their ability to produce phenoloxidases capable of degrading phenolic compounds. The fungi were inoculated in media containing different concentrations of gallic acid, incubated at 28℃ and monitored every 48 h. Enzyme production was assessed through the observation of an amber-colored halo, which is characteristic of the Bavendamm’s reaction. Only Fusarium sp. strain D3-FB and Cercospora sp. strain D7-FB showed degradation halos at all concentrations. Although developed in the same media, the other species showed no signs of the Bavendamm’s reaction.
Keywords: Phenoloxidases, Baccharis Dracunculifolia, Phenolic Compounds, Endophytic
Cite this paper: Sideney Becker Onofre , Paula Steilmann , "Phenoloxidases Produced by Endophytic Fungi Isolated from Baccharis Dracunculifolia D. C. (Asteraceae)", Resources and Environment, Vol. 2 No. 6, 2012, pp. 271-274. doi: 10.5923/j.re.20120206.04.
Article Outline
1. Introduction
- Industrial processes are a major cause of environmental problems. However, it is through industrialization that food and other essential items are obtained. As industry is of paramount importance, an alternative to solve and reduce the problems caused to the environment is increasingly sought-after[1].Large-scale industrialization, increase in production, large population concentration in certain regions and intense agricultural activity contribute to the significant rise in waste discharges into watercourses. The improper release of solid, liquid and gas wastes from different sources changes the characteristics of soil, water and air, and it may cause environmental pollution or contamination. Pollution occurs when these residues alter the aesthetic aspect, the composition or the shape of the physical environment, while the environment is considered contaminated when there is a minimal threat to human, animal and plant health.Bioremediation is a technology that uses microorganisms to reduce or remove petroleum hydrocarbons pollutants from the environment. This technology can be defined as a reaction biologically catalyzed that transforms chemically complex compounds into simpler substances. In the case of organic compounds, there may be conversion of the original constituents into inorganic substances, a process called mineralization[2].A variety of organic substances found in oil refinery and industrial effluents are efficiently degraded by bacteria, yeasts and filamentous fungi, due to their ability to use such substances as a carbon and energy source. Such ability makes these organisms an increasingly useful alternative to conventional methods of treatment in the solution of environmental problems[3].According to Bentro, Camargo and Okeke[4], there are different bioremediation strategies: natural or intrinsic bioremediation, by using autochthonous microorganisms without any interference of active remediation technologies; biostimulation, by adding stimulating agents, such as nutrients, oxygen and biosurfactants; and bioaugmentation, through the inoculation of enriched microbial consortia. The purpose and advantage of these techniques is the mineralization of the pollutant.Fungi secrete a large variety of enzymes, which are effective when assisting in their nutrition, thus being responsible for the deterioration of various natural, refined or processed materials[5]. In recent decades, the use of filamentous fungi and their metabolites in bioremediation processes has been growing, due to the high potential of biosorption (heavy metals and dyes), degradation and efficient mechanisms of resistance to adverse environmental conditions. Among the important industrial enzymes produced by fungi are cellulases, phenoloxidases and tannases[6].The survey and characterization of new strains with biodegradative ability, adaptability to local environmental conditions and tolerance to toxicity are important aspects yet to be explored in a program that aims to apply bioremediation processes[7].Another issue to be considered is the wide variety of pollutants, including phenols, released by industries. Although these compounds are found in nature, they are a major residual toxic pollutant, mainly discarded by the petrochemical and textile industries[8].Many substances considered harmful, such as phenolic compounds, can have their toxicity reduced or eliminated through the action of adapted microorganisms. Environmental conditions, including temperature, pH, oxygen and salinity, can promote or suppress the degradation of these compounds[6].Some fungi are able to produce phenoloxidases in media containing phenols, through a process called Bavendamm’s reaction. Under the action of fungal phenoloxidases, gallic acid forms quinones, which are identified by the formation of an amber-colored halo around the mycelium[2]. The production of phenoloxidases or polyphenol oxidases is extremely important in biotechnology, as these enzymes are responsible for breaking the phenolic groups present in industrial effluents and petroleum hydrocarbons. Microorganisms that produce these enzymes can be easily used in decontamination of polluted environments[9].This study sought to evaluate the ability of seven species of endophytic fungi isolated from Baccharis dracunculifolia D.C. (Asteraceace) in producing phenoloxidases and degrading phenolic compounds.
2. Materials and Methods
2.1. Fungi Assessed
- The research was conducted in the years 2008 and 2009 with seven species of endophytic fungi isolated from Baccharis dracunculifolia D.C. (Asteraceae), which were maintained in the mycology collection of the Microbiology Laboratory of the University of Parana (UNIPAR), Francisco Beltrão Campus, PR, Brazil. The species used are: Aspergillus sp. strain D2-NC, Fusarium sp. strain D3-FB, Colletotrichum sp. strain D4-FB, Acremonium sp. strain D5-FB, Cercospora sp. strain D7-FB, Cylindrocladium sp. strain D8-FB and Phomopsis sp. strain D10-NC.
2.2. Culture Medium
- The species were evaluated on a minimal medium (MM), containing NaNO3 (0.38 g/L), KH2PO4 (1.19 g/L), MgSO47H2O (0.50 g/L), KCl (0.50 g/L), FeSO47H2O (0.01 g/L), agar (20 g/L), supplemented with different concentrations of gallic acid (0.80, 1.0, 1.20, 1.40, 1.60 and 1.80 g/L).
2.3. Degradability
- Inoculation was performed on each plate from a sample kept on a PDA culture medium, then incubated at 28℃ and monitored every 48 h for a period of 240 h, when the amber-colored halo characteristic of the Bavendamm’s reaction occurred. Under the action of fungal phenoloxidases, gallic acid forms quinones, which are identified through the halo formed around the mycelium[2]. Evaluations were conducted every 48 h and each colony was measured with a millimeter ruler in two diametrically opposite directions to obtain the growth mean in the period.
3. Results and Discussion
- The ability to produce phenoloxidases and degrade phenolic compounds of the studied species are shown in Table 1.According to data shown in Table 1, Fusarium sp. strain D3-FB had the best behavior in the degradation of phenolic compounds (represented in this study by gallic acid), followed by Cercospora sp. strain D7-FB. The other five species studied grew on a minimal medium, but the formation of degradation halos of gallic acid by the action of phenoloxidases was not observed (Figure 1). This behavior is shown in Table 1, through positive BR or negative BR.
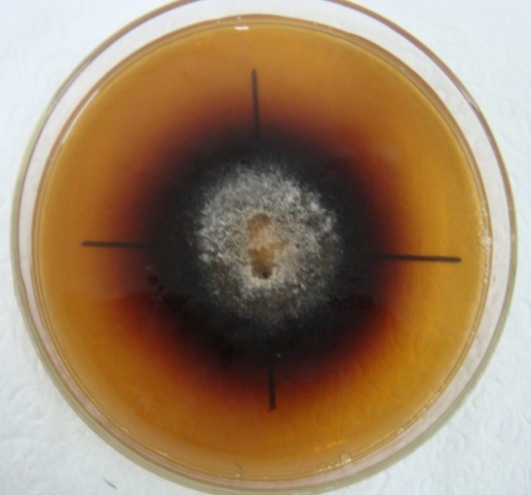 | Figure 1. Characteristics of the Bavendamm’s reaction caused by the oxidation of gallic acid and the formation of quinones on a Fusarium sp. strain D3-FB culture |
|
4. Conclusions
- Fusarium sp. and Cercospora sp. can be used as polyphenoloxidase producers, because the Banvedamm’s reaction could be observed during this study. All studied species developed on a minimal medium containing gallic acid, but the fungus Phomopsis sp. can only be placed in environments with concentrations equal to or lower than 0.80 g/L. However, Acremonium sp. may be introduced in environments with concentrations equal to or lower than 1.0 g/L. Fusarium sp. strain D3-FB showed the highest production rates of polyphenol oxidase observed through the Bavendamm’s reaction, because it grew on all concentrations of phenol tested.
ACKNOWLEDGEMENTS
- The authors thank the Paranaense University (UNIPAR) for the financial support, through the Program of Research Funding, proclamation 2011, and the Brazilian National Council for Scientific and Technological Development (CNPq) through the Program for Scientific Initiation Scholarships - PIBIC.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML