-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2012; 2(4): 175-179
doi: 10.5923/j.re.20120204.07
Comparative Solubility Study of Four Phosphatic Fertilizers in Different Solvents and the Effect of Soil
P. K. Ghosal 1, T. Chakraborty 2
1Agricultural and Ecological Research Unit,, Indian Statistical Institute, Kolkata, 700108, India
2Palli Siksha Bhavana, Visva Bharati University, Bolpur, Postcode, India
Correspondence to: P. K. Ghosal , Agricultural and Ecological Research Unit,, Indian Statistical Institute, Kolkata, 700108, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Phosphate rocks (PRs) are suitable for direct application as a possible alternative to more expensive soluble phosphate fertilizers in agricultural fields. But the ability of the PRs to release phosphates in the plant available forms depends on the particle size and chemical and mineralogical characteristics of the PRs as well as the properties of the soil in which they are applied. So an experiment was conducted with four sources of phosphatic fertilizers namely Triple super phosphate (TSP – 21.75%P)), Partially acidulated phosphate rock (PAPR – 12.97%P)), Morocco rock phosphate (MORP – 14.87%P)) and Mussoorie rock phosphate (MRP – 8.12%P) whose solubility were tested in six different extractants namely 2% Citric acid, 0.002N Hydrochloric acid, N-Ammonium citrate, Bray-2P extractant, Olsens’s extractant and Morgan’s reagent under seven periods of incubation (1, 2, 3, 7, 10, 15 and 30 days), with and without soil. The results revealed that release of P were increased on addition of soil irrespective of fertilizers or extractants used. TSP released maximum P (3.05% - 3.27% with soil, 2.11% - 2.22% without soil) by the 7th day of incubation. The partially acidulated source was found to release P, higher than rock phosphates but lower than TSP, for the initial periods of incubation (1-3 days) (1.31%-1.34% with soil, 0.46% without soil) with an increase in the later periods (7th day onward) (1.27%-1.92% with soil, 0.55%-0.66% without soil). The PRs released maximum P after the 7th day of incubation. Among the different solvents, maximum release of phosphorus was observed by 2% citric acid followed by Bray 2P and Olsen’s extractants.
Keywords: Phosphate Rocks, Acidulated Rock Phosphates, Solubility in Different Extractants
Article Outline
1. Introduction
- Phosphate rocks (PRs) are suitable for direct application to agricultural fields under certain conditions[1] due to their fairly open, loosely consolidated aggregates ofmicro -crystals with relatively large surface areas. Direct application of phosphate rock to soil as a possible alternative to the more expensive soluble phosphate fertilizers in tropical cropping system has received considerable attention in recent years[2]. The principal mineral in most PR sources is apatite, but it varies widely in physical, chemical, and crystallographic properties[3]. The solubility of PR reflects the chemical and mineralogical characteristics of the specific P minerals. Naturally occurring PRs differs widely in their mineralogy and their chemical reactivity or solubility is a measure of the PR’s ability to release phosphorus (P) for plant uptake. Gholizadeh et al[4] reported that to avoid time, trouble and cost of doing field trials for determining the reactivity of PRs, solubility of these in citric acid could be a criterion for predicting their reactivity.The effectiveness of phosphate rock relative to soluble P fertilizer will vary from source to source depending upon the mineralogy and chemistry of each rock as well as the influence of soil, crop, environment and management factors. According to Rajan et al[5] reactivity is ‘the combination of PR properties that determines the rate of dissolution of the PR in a given soil under given field condition. The reactivity of PRs, the main constituent of which is mineral apatite (Ca5(PO4)3X) where X is predominantly fluorine, is determined by the rate of dissolution in acid and the amount of P recovery. The main factors affecting the reaction rate of acid attack of phosphate are acid concentration, time of the reaction, solid/liquid ratio, particular size and temperature[6]. The reactivity also depends on the composition of the apatite mineral, presence of impurities and particle size. Increasing degree of substitution of carbonate forphosphate, and of magnesium and sodium for calcium in the apatite structure and decreasing particle size enhance the reactivity of PRs[7]. It is also known that decreasing soil pH increases PR effectiveness[8],[9],[3] and rock phosphatedissolution was also shown to be linearly correlated with the reserve acidity of the soil. Asomaning et al[10] reported that the two major factors generally recognized as influencing P availability from phosphate rocks are: (i) inherentdifferences among PR sources, and (ii) soil properties. The release of P from PR generally increases with a greater P-fixing capacity of the soil[3]. Thus soil has an active role in dissolution of PRs and releasing P into the soil solution for plant uptake.With this background an experiment was set in the laboratory of Agricultural and Ecological Research Unit of Indian Statistical Institute, Kolkata, to study the solubility of four phosphatic fertilizers – Triple super phosphate (21.75% P), Morocco rock phosphate (14.87% P), Musssoorie rock phosphate (8.12% P) and Partially acidulated rock phosphate (12.97% P) in six different solvents namely 2% citric acid, 0.002N hydrocholric acid, N-Ammonium citrate, Bray-2P extractant, Olsen’s extractant and Morgan’s reagent.
2. Materials and Methods
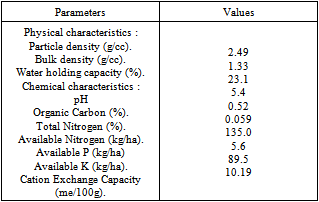
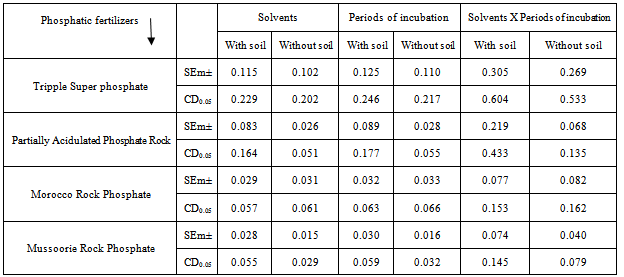
- The experiment was carried out with each fertilizer for 7 periods of incubation (1, 2, 3, 7, 15 and 30 days), with and without soil, under factorial randomised block design in three replications. The soil added was acid lateritic and rich in iron and aluminium. The physico-chemical properties of the soil under experimentation are given in Table 1.
|
2.1. Treatment Details
2.2. Extraction
- Ten gram (10 g) of air dried, 2 mm sieved soil, was taken in 100 ml conical flask and to it 20 mg of desired phosphatic fertilizer was added. Then 50 ml of the desired solvent was added and the flasks were shaken on a mechanical shaker for 15 minutes and kept in a biochemical/biological oxygen demand (BOD) incubator at 300C for different periods of time. BOD are suitable for closed control incubation and are also called low temperature incubator. After the specified periods as per treatment, each flask was taken out of the BOD and the materials were filtered. An aliquot of 5 ml was taken per treatment and its phosphate (available P) content was determined following the standard procedure using the ammonium meta vanadate[11] solution.In another set of experiment, the same procedure was followed for extraction of phosphate from the phosphatic fertilizers as mentioned above but without adding soil to determine the role of soil, if any, in the method of extraction of phosphorus.
3. Results and Discussions
- The data on P- solubility revealed that among P sources, the water soluble and partially soluble sources released almost double the amount of P, with soil than that without soil, for all the extractants (Figures 1,2). This can be explained due to the fact that in the treatments with soil, the readily available P, reacts with the Fe and Al ions in the acid soil thereby forming iron and aluminium phosphate complex as intermediate products which are also extracted by the extractants along with the fertilizers. An increase in the P solubility of the rock phosphate source was also noted as affected by the soil. Rajan et al[5] expressed dissolution of rock phosphate in acid soils as, -Ca10(PO4)6F2 + 12H2O
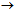 10Ca+2 + 6H2PO4- + 2F- +12OH-Although the above reaction was for fluorapatite, it applies to other members of the apatite minerals including reactive rock phosphates (francolites). The increase in dissolution of the rock phosphates when treated with soils may also be attributed to the neutralization of the OH- ions, released on hydrolysis of the phosphate ions, due to soil acidity. The solubility of P may influence PR dissolution products since the P released from PRs is effectively removed from solution by iron and aluminium oxides, thus, creating a gradient for further dissolution of the PR[13]. Chhonkar[14] also reported that P availability to plants was significantly increased by the action of soil. It was interesting to note that at 15 and 30 days of incubation, P released from TSP, without soil was more (1.94%,1.43%) than that with soil (1.68%,0.84%) (Table 3, Figure 1- a,b). This can be ascribed to adsorption of soluble P from soil solution by the reprecipitated poorly crystalline ferrous hydroxides or carbonates from Fe+2 ions formed by soil reduction ([15],[16],[17]).The water soluble source (TSP) was found to release the maximum P (3.05%-3.27% with soil, 2.11%-2.22% without soil) by the 7th day of incubation where as the rock phosphate sources released maximum amount of P after the 7th day (Fig 1,2). The partially acidulated source was found to release P, higher than rock phosphates but lower than TSP, for the initial periods of incubation (1-3 days) (1.31%-1.34% with soil, 0.46% without soil) with an increase in the later periods (7th day onward) (1.27%-1.92% with soil, 0.55%-0.66% without soil) (Table 4, Figure 1 – c,d). This is obviously due to the presence of partly water soluble P which was released earlier. The trend of P-release by the fertilizers was more pronounced for the treatments with soil. The results on P release thus showed that maximum release of P from the unacidulated and partially acidulated rock phosphates needs some more periods of incubation for thorough acidulation of the fertilizer with the extractant to come into equilibrium with P in solution. Singh et al[18] and Barnes and Kamprath[19] also reported an increase in P-availability with the length of incubation period and opined that it may take 4 to 8 weeks for phosphate rocks to reach their maximum solubility. Rajan et al[20] and Sinclair et al[21] explained that this is due to the insoluble characteristic of the phosphate rock where a time lag is experienced forunacidulated rock phosphates to reach the maximumeffectiveness .Further , among the different solvents, maximum release of phosphorus was observed by 2% citric acid followed by Bray 2 and Olsen’s extractants from TSP, PARP, MORP and MRP, with and without soil. Similar observations was also reported earlier by[12]. Higher solubility of the PRs in 2% citric acid possibly results from higher reactivity rather than from any difference in surface area presented for dissolution[22]. Among the two rock phosphate sources, MORP was found to release greater amount of P than MRP. This can be attributed to larger CO3-2 substitution for PO4-2 in MORP (5.60% CO2) crystal lattice which renders MORP to be unstable and more reactive than MRP, which is also a francolite (carbonate apatite) but with a smaller degree of carbonate substitution[23]. Rajan et al[5] opined that apatites are not soluble in bicarbonate solutions and therefore Olsen’s extractant cannot be expected to predict potential P-release from rock phosphates. But the perusal of the solubility data (Figure 1,2) revealed that Olsen’s extractant showed more P than some acidic extractants, both in treatments with and without soil. A probable explanation for this may be the presence of Al-P and Fe-P in the rock phosphate crystals and in the intermediate products of the soil-PR interaction, from where, alkaline Olsen’s extractant may dissolve potentially unavailable P and thus overestimate P[5].The study on the solubility pattern of the water soluble triple super phosphate, two rock phosphates – Morocco and Mussoorie and one partially acidulated rock phosphate thus suggested the order of solubility of P or its reactivity as TSP>PARP>MORP>MRP. Hammond et al[23] reported, however, that the effectiveness of a P source measured under actual field condition will vary with changes in a number of climatic and agro-edaphic conditions. Thus, their mean value of soluble P from the treated fertilizers with soil (TSP, PARP, MORP and MRP) varied from 3.27 to 0.84%, 1.92 to 1.27%, 0.81 to 0.31% and 0.47 to 0.6% respectively.
10Ca+2 + 6H2PO4- + 2F- +12OH-Although the above reaction was for fluorapatite, it applies to other members of the apatite minerals including reactive rock phosphates (francolites). The increase in dissolution of the rock phosphates when treated with soils may also be attributed to the neutralization of the OH- ions, released on hydrolysis of the phosphate ions, due to soil acidity. The solubility of P may influence PR dissolution products since the P released from PRs is effectively removed from solution by iron and aluminium oxides, thus, creating a gradient for further dissolution of the PR[13]. Chhonkar[14] also reported that P availability to plants was significantly increased by the action of soil. It was interesting to note that at 15 and 30 days of incubation, P released from TSP, without soil was more (1.94%,1.43%) than that with soil (1.68%,0.84%) (Table 3, Figure 1- a,b). This can be ascribed to adsorption of soluble P from soil solution by the reprecipitated poorly crystalline ferrous hydroxides or carbonates from Fe+2 ions formed by soil reduction ([15],[16],[17]).The water soluble source (TSP) was found to release the maximum P (3.05%-3.27% with soil, 2.11%-2.22% without soil) by the 7th day of incubation where as the rock phosphate sources released maximum amount of P after the 7th day (Fig 1,2). The partially acidulated source was found to release P, higher than rock phosphates but lower than TSP, for the initial periods of incubation (1-3 days) (1.31%-1.34% with soil, 0.46% without soil) with an increase in the later periods (7th day onward) (1.27%-1.92% with soil, 0.55%-0.66% without soil) (Table 4, Figure 1 – c,d). This is obviously due to the presence of partly water soluble P which was released earlier. The trend of P-release by the fertilizers was more pronounced for the treatments with soil. The results on P release thus showed that maximum release of P from the unacidulated and partially acidulated rock phosphates needs some more periods of incubation for thorough acidulation of the fertilizer with the extractant to come into equilibrium with P in solution. Singh et al[18] and Barnes and Kamprath[19] also reported an increase in P-availability with the length of incubation period and opined that it may take 4 to 8 weeks for phosphate rocks to reach their maximum solubility. Rajan et al[20] and Sinclair et al[21] explained that this is due to the insoluble characteristic of the phosphate rock where a time lag is experienced forunacidulated rock phosphates to reach the maximumeffectiveness .Further , among the different solvents, maximum release of phosphorus was observed by 2% citric acid followed by Bray 2 and Olsen’s extractants from TSP, PARP, MORP and MRP, with and without soil. Similar observations was also reported earlier by[12]. Higher solubility of the PRs in 2% citric acid possibly results from higher reactivity rather than from any difference in surface area presented for dissolution[22]. Among the two rock phosphate sources, MORP was found to release greater amount of P than MRP. This can be attributed to larger CO3-2 substitution for PO4-2 in MORP (5.60% CO2) crystal lattice which renders MORP to be unstable and more reactive than MRP, which is also a francolite (carbonate apatite) but with a smaller degree of carbonate substitution[23]. Rajan et al[5] opined that apatites are not soluble in bicarbonate solutions and therefore Olsen’s extractant cannot be expected to predict potential P-release from rock phosphates. But the perusal of the solubility data (Figure 1,2) revealed that Olsen’s extractant showed more P than some acidic extractants, both in treatments with and without soil. A probable explanation for this may be the presence of Al-P and Fe-P in the rock phosphate crystals and in the intermediate products of the soil-PR interaction, from where, alkaline Olsen’s extractant may dissolve potentially unavailable P and thus overestimate P[5].The study on the solubility pattern of the water soluble triple super phosphate, two rock phosphates – Morocco and Mussoorie and one partially acidulated rock phosphate thus suggested the order of solubility of P or its reactivity as TSP>PARP>MORP>MRP. Hammond et al[23] reported, however, that the effectiveness of a P source measured under actual field condition will vary with changes in a number of climatic and agro-edaphic conditions. Thus, their mean value of soluble P from the treated fertilizers with soil (TSP, PARP, MORP and MRP) varied from 3.27 to 0.84%, 1.92 to 1.27%, 0.81 to 0.31% and 0.47 to 0.6% respectively. 5. Conclusions
- It can thus be concluded that Morocco and Mussoorie phosphate rocks can be efficiently used as fertilizers in acid lateritic soils but have to be applied a bit earlier before crop cultivation as it needs some time for releasing P into the soil solution. But water soluble and partially acidulatedphosphate rock can release P immediately for crop uptake. Solubility measurement cannot be used to predict specific yield response but they can serve as a useful means of predicting relative performance of one source to another and this assist in selection of the most appropriate source.
|
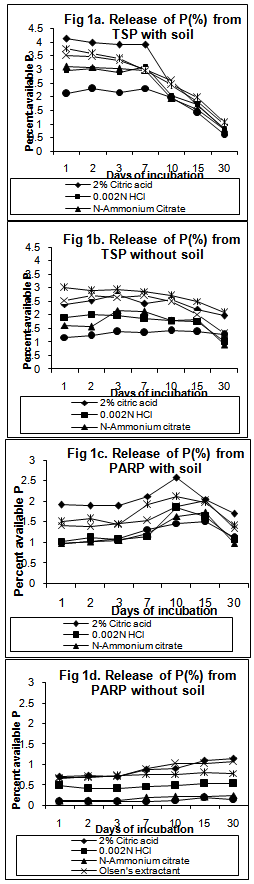 | Figure 1. Release of available P from TSP and PARP by different solvents at different periods of incubation |
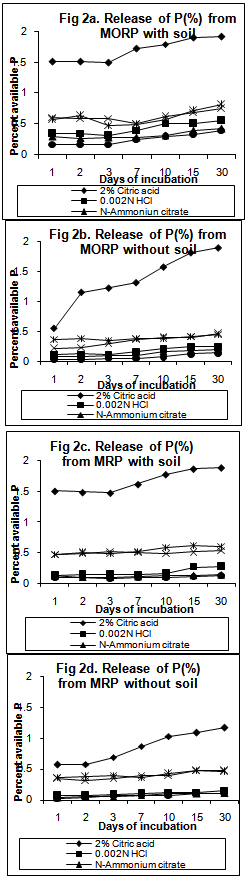 | Figure 2. Release of available P from MORP and MRP by different solvents at different periods of incubation |
References
| [1] | Zapata F, FAO/IAEA research activities on direct application of phosphate rocks for sustainable crop production. In: SSS Rajan and SH Chien eds “Direct application of phosphate rock and related technology; latest developments and practical experiences”, Proc. Int. Meeting, Kuala Lumpur, 16 – 20 July 2001, Muscle Shoals, USA, IFDC, pp. 441, 2003. |
| [2] | Akande M O, Makinde E A, Oluwatoyinbo F I and Adetunji T, “ Effects of phosphate rock application on dry matter yield and phosphorus recovery of maize and cowpea grown in sequence”, African Journal of Environmental Science and Technology, vol. 4 no. 5, pp. 293-303, 2010. |
| [3] | Chien S H, Prochnow Luis I and Mikkelsen R, “Agronomic Use of Phosphate Rock for Direct Application” Better Crops vol. 94, no.4, pp.21-23, 2010. |
| [4] | Gholizadeh A, Ardalan M, Tehrani M M, Hosseini H M, Karimian N, “Solubility test in some phosphate rocks and their potential for direct application in soil”, World Applied Sciences Journal vol.6, no.2, pp.182-190, 2009. |
| [5] | Rajan, S S S, Watkinson J H and Sinclair A G, “Phosphate rocks for direct application to soils” Advances in Agronomy, vol.57, pp. 77-159, 1996. |
| [6] | Hamadi A S, Remedhan ST and Ali H A, “Phosphate rock treatment with hydrochloric acid for increasing P2O5 content”, Eng. And Tech Journal, vol. 30, no. 1, pp. 67-76, 2012. |
| [7] | Chien S H and Hammond L L, “A comparison of various laboratory methods for predicting the agronomic potential of phosphate rock for direct application”, Soil Science Society of America Journal, vol.42, pp. 1758-1760, 1978. |
| [8] | Prochnow L I, Quespe J F S, Francisco E A B and Braga G, “Effectiveness of phosphate fertilizers of different water solubilities in relation to soil phosphorus adsorption”, Scientia Agricola, vol. 63, no. 4, 2006. |
| [9] | Rivaie A A, Loganathan P, Graham J D, Tillman R W and Payn T W, “Effect of phosphate rock and triple superphosphate on soil phosphorus fractions and their plant availability and downward movement in two volcanic ash soils under Pinus radiate plantations in New Zealand”, Nutr Cycl Agroecosyst, vol.82, pp.75-88, 2008. |
| [10] | Asomaning S K, Abekoe M K and Owusu-Bennoah E, “Phosphate rock dissolution and availability in some soils of semi-deciduous rainforest zone of Ghana”, West African Journal of Applied Ecology, vol.10, no. 1, 2006. |
| [11] | Jackson M L, In : Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd. New Delhi, 1972. |
| [12] | Chakraborty T and Chattopadhyay S D, “Effect of different solvents and periods of incubations on the available phosphate content of Purulia rock phosphate”, Indian Agriculturist, vol. 31, no. 2, pp. 135-140, 1987. |
| [13] | Smyth J J and Sanchez P A, “Phosphate rock dissolution and availability in Cerrado soils as affected by Psorption capacity”, Soil Science Society of America Journal, vol. 46, pp. 330–345, 1982. |
| [14] | Chhonkar, P K, “Mobilisation of soil phosphorus through microbes : Indian experience. Phosphorus research in India” Proceeding of group discussion held at IARI, New Delhi, 10th December, 1993, pp.120-125, 1994. |
| [15] | Patrick W H, Jr. Mikkelsen D S and Wells B R, “Plant nutrient behaviour in flooded soil” In: Fertilizer Use and Technology, 3d ed. Soil Science Society of America, Madison Wisconsin, pp. 197-228, 1985. |
| [16] | Ponnamperuma F N, “Chemical kinetics of wetland rice soils relative to soil fertility” In : Wetland Soils : Characterisation, Classification and Utilisation, International Rice Research Institute, Los Banos, Laguna, Philippines, pp .71-89, 1985. |
| [17] | Tian-ren Y, Kirk G I D and Chaudhury F A, “Phosphorus chemistry in relation to water regime” Proceedings of symposium on Phosphorus Requirements for Sustainable Agriculture in Asia and Oceania, 6-March, International Rice Research Institute, Los, banos, Laguna Philippines, 1989. |
| [18] | Singh D, Mannika N D and Srivas N C, “Fertilizer value of indigenous rock phosphates compared with single super phosphate : Laboratory incubation studies with farm yard manure”, Journal of Indian Society of Soil Science, vol.24 no.1, pp. 78-80, 1976. |
| [19] | Barnes J S and Kamprath E J, “Availability of North Carolina rock phosphate applied to soils”, North Carolina Agricultural Station Technical Bulletin. pp . 229, 1975. |
| [20] | Rajan S S S, Gillingham A G O, O’Connar, M B, Percival N A and Gray M G, “Ground phosphate rocks as fertilizers for pastures” In : The use of reactive phosphate rocks and their derivatives as fertilizers. Ed. by White, R. E. and Currie, L. D. Occassional Report No. 1, Massey University, Palmerston North, New Zealand, pp .78-83, 1987. |
| [21] | Sinclair A G, Johnstone P D, Smith L C, O’Connor M B and Nguyen L, “Agronomy, modeling and economics of reactive phosphate rocks as slow release phosphate fertilizers for grasslands”, Fertilizer Research, vol. 36, pp. 229-238, 1993. |
| [22] | Syers J K, Mackay A D, Brown M W, Currie L D, “Chemical and physical characteristics of phosphate rock materials of varying reactivity”,Journal of the Science of Food and Agriculture, vol.37, no. 11, pp.1057–1064, 1986. |
| [23] | Hammond L L, Chien S H and Mokwunye A U, “Agronomic value of unacidulated and partially acidulated phosphate rocks indigenous to the tropics”, Advances in Agronomy, vol. 40, pp.89-140, 1986. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML