-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2012; 2(2): 20-29
doi: 10.5923/j.re.20120202.04
Groundwater Quality Assessment of Yenagoa and between 2010 and 2011
Gordon T. Amangabara 1, Enyinaya. Ejenma 2
1Dept of Environmental Technology, Federal University of Technology, Owerri; Nigeria
2Federal College of Agriculture, Ishiagu, Ebonyi State, Nigeria
Correspondence to: Gordon T. Amangabara , Dept of Environmental Technology, Federal University of Technology, Owerri; Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
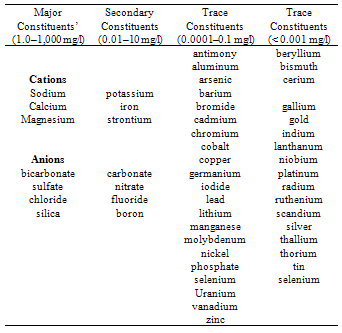
The hydrochemical characteristics of groundwater in Yenagoa and environs was investigated to determine the areal distribution of geochemical parameters and attempt to explain the controlling processes responsible for the various facies. Sixty water samples were collected from wells ( Borehole) at different times in both the dry season ( December to February) and the raining/wet Season ( Between May and October) and were analyzed for various parameters including pH, total dissolved solids (TDS), potassium (K), Sodium (Na), Calcium (Ca), Magnesium (Mg), Chlorine (Cl), Bicarbonate (HCO3) and sulphate ion (SO42-) after standard procedures. The Data obtained from the laboratory analysis were used as variable inputs for the descriptive statistics such as mean, minimum, maximum and standard deviation. Laboratory tests were carried out on representative soil samples in accordance with British Standards (B.S) 1377, which are equivalent to the American Society of Testing Materials (ASTM) standards. The tests were grain size distribution analysis, permeability (using the constant and falling head permeameters) and Atterberg (consistency) limits and was conducted to enable the evaluation of the gradation, hydraulic conductivity (coefficient of permeability/ and consistency (water absorbing and adsorbing ability) properties of the soil samples, as well as their classification. Results showed that water table was generally close to the surface of the area. The first 0 to 5cm depth consist of organic clay, and essentially, the area is mostly characterized by the sandy – clay and silty-clay materials. By implication, the upper clay protective layer for groundwater is thin, coupled with organic materials, biochemical oxygen demand is enhanced. Permeability and transmissivity character of the borehole indicate that if surface water is polluted in one area it can easily get transferred to other part of Yenagoa thus causing serious concern for health implication. The physico-chemical character of the groundwater reflects the hydrochemical facie of the groundwater in the study area. The most prevalent ionic concentration includes iron, calcium, magnesium, Sodium, potassium, chloride, and bicarbonate. The trend of occurrence shows the following relationship: Ca > HCO3 > Na+ > K > Mg > Cl- > SO42. Na+ > K > Cl-. There is elevated occurrence of Ca2+ > HCO3- > Mg2+ ions in the groundwater beyond permissible level reflecting processes of natural rainwater recharge and water – soil/rock interaction
Keywords: groundwater, hydrochemical, borehole, physicochemical, pollution, aquifer
Article Outline
1. Introduction
- The challenge of ensuring usable water in sufficient quantities to meet the needs of human and ecosystems emerged as one of the primary issues of the 21st century (Eden and Lawford, 2003). For example, inadequate water supply and poor water quality give rise to health and other societal issues, limit agricultural productivity and economic prosperity, and pose national security risk in some countries (Lawford et al., 2003; Nwidu et al., 2008). Problem of this nature have been increasing in scope, frequency, and severity because the demand for water continue to grow while supply of renewable water remain fixed. While it is agreed that water is one of the most important resources with great implications for African development, the freshwater situation in is unfortunately not encouraging. Presently, it is estimated that more than 300 million people in live in a water-scarce environment. By 2025, eighteen African countries are expected to experience water stress. The amount of freshwater available for each person in is about one-quarter of what it was in 1950. In many countries, requirements for domestic freshwater use, sanitation, industry and agriculture cannot be met (African Water Journal). The situation is getting worse as a consequence of population growth, rapid urbanization, increasing agriculture and industrial activities, and lack of adequate capacity to manage freshwater resources.Bayelsa (Figure 1.) is a wetland in the Niger Delta with possible serious problems of drinking water. Bayelsa State Government had reported that all the surface water bodies in the State are pollution-laden and hence there is the need to resort to groundwater for consumable water (Bayelsa State Water Board, 2001). Water enters the subsurface through infiltrating precipitation and through percolation from surface water bodies. Once in the groundwater systems, water moves through the subsurface, at varying rates, in response to pressure and elevation differences and permeability of the geologic materials through which it travels. As a prelude to understanding and predicting the impact of future water development plans in the quality of water in the area, it is necessary to investigate and evaluate the hydrochemistry of the groundwater. More so, that the surface of the delta is dissected by a dense network of rivers and creeks, which create a condition of delta-wide hydrological continuity. Water pollution in one part of the delta can readily be felt in other part.
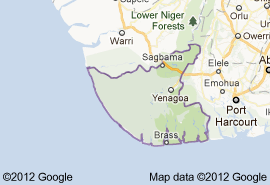 | Figure 1. Map of State |
2. Materials and Method
- The Study AreaYenagoa, the study area is the capital of . It lies within latitudes 04°4N and 05°, 02N and longitudes 006°, 15E and 006°, 24E and situated in the southern part of the Niger Delta of Nigeria (Figure 1). It is underlain by a sequence of sedimentary formations with a thickness of about 8000 metres and include from bottom to top, Akata Formation, Agbada Formation, Benin Formation. The Benin Formation is Oligocene to Pleistocene in age. It consists predominantly of freshwater continental friable sands and gravel that are of excellent aquifer properties, with occasional intercalation of shales. This formation contains the most productive and hence most tapped aquifer in the delta region especially in areas of Yenagoa where it is shallow. It consists of fine to medium and coarse-grained unconsolidated sands that are often feldspathic (with 30-40 wt % feldspars) and occasionally gravelly. The formation generally does not exceed 120 metres in thickness and it is predominantly unconfined.The hydraulic conductivities of the sand vary from 3.82 x 10-3 to 9.0 x 10-2 cm/sec, which indicates a potentially productive aquifer. Specific capacities recorded from different areas within this formation vary from 6700 lit/hr/m to 13,500 lit/ hr/m (Offodile, 1991). The water table is very close to the ground surface and varies from 0 to 4 metres. This limited groundwater level fluctuation reflects the high amounts of precipitation often recorded in Yenagoa over the greater part of the year. Recharge is from precipitation and the numerous rivers in the delta. Fresh water occurs in shallow unconfined aquifers, in sands of the coastal beach ridges and river point bars, as well as in sandy islands within the mangrove belt. It also occurs in confined aquifers at varying depths.
|
3. Methodology
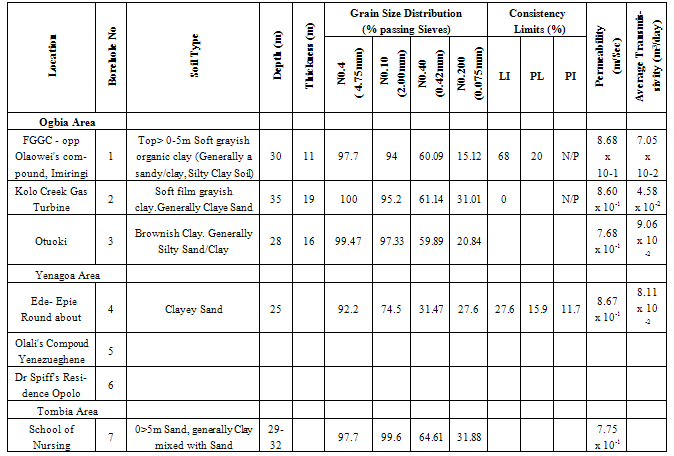
- Water samples from seven different sources were collected during a twelve-month period, Table 1 shows the locations.Water Samples were collected from already sunk private bore holes in two seasons. The dry season measurements were taken on the 15th of every month between November - December 2010 and January to March 2011 and Raining Season measurements taken on the 15th of every month between June and October 2010) The borehole characteristics i.e. depth, soil property, yield etc where available was also collected from the owners of the boreholes sampled.The samples for physicochemical investigationScrew capped sterile bottles were used to collect water samples from the boreholes, which have hand pumps or tap outlets. The hand pumps were operated continuously for five (5) minutes before samples were taken aseptically for analysis as done by Cheesebrough, (1991) and Ogbonna et al., (2007). The collected sample was then labeled at the point of collection, preserved and stored prior to taking them to the laboratory. Bottles were already rinsed with water to be sampled before sample collection. Sufficient air space was left to create space for water expansion. This mode of data collection is called Ruthner sampling method. One advantage of this method is that it provides immediate knowledge of the water temperature at the same time of collection. Some fast changing physicochemical parameters e.g. Dissolved Oxygen, were determined in situ with the aid of portable coleman pH meter, salinity, temperature, Total Dissolved Solids (TDS) and conductivity were also determined in situ with the aid of conductivity/TDS meter (Jackson, 1962, APHA, 1985)Laboratory TechniquesTotal Hardness of the water was obtained by titrimetric methods. 25ml of sample was diluted with distilled water after which, 2 drops of buffer solution (pH 10), sodium cyanide (250g) and indicator powder (200g) were added. The solution was then titrated with 0.01m to a blue end point. The hardness was obtained as mg CaCO3 per litre. Alkalinity was obtained by titrating 50ml of sample with HCl (0.1m) while turbidity was estimated by nephelometric method using La Motte 2020 portable turbidity meter. The Data obtained from the laboratory analysis were used as variable inputs for the descriptive statistics such as mean, minimum, maximum and standard deviation.
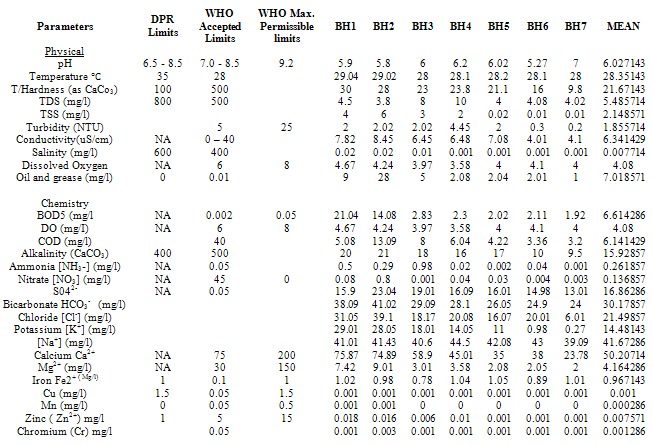
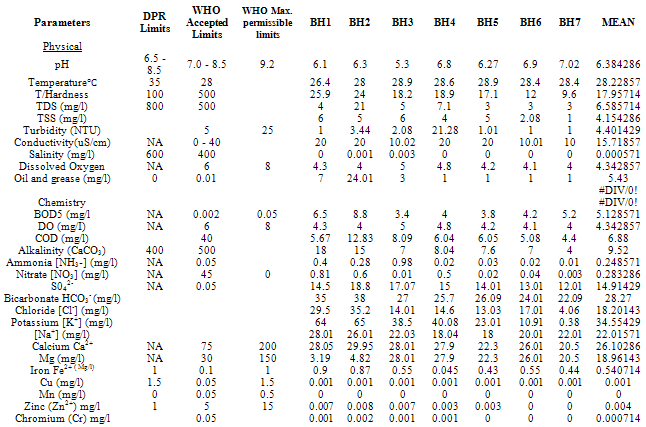
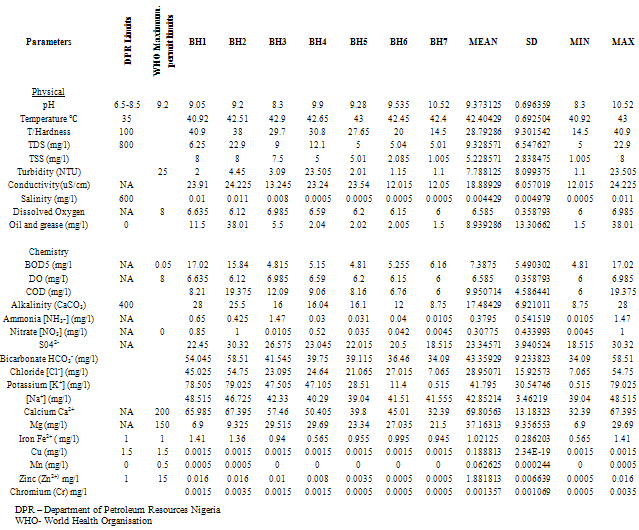
4. Results and Discussion
- Results obtain from both field work and laboratory analyses are presented as Table 2 – 5.
|
|
|
|
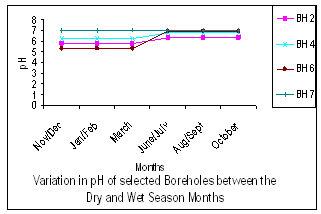 | Figure 2. Variation of pH between Dry and Wet seasons |
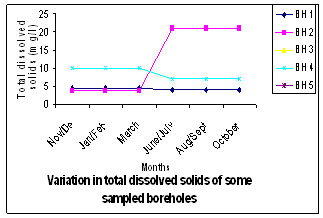 | Figure 3. Variation in TDS in Boreholes Sampled |
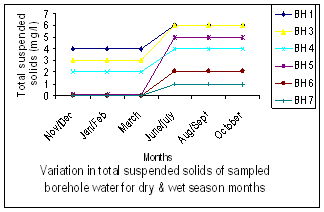 | Figure 4. Variation in Total Suspended Solids |
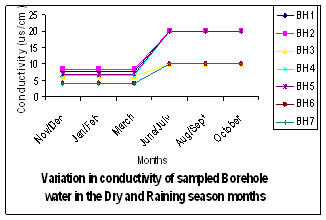 | Figure 5. Variations in Conductivity of Sampled Boreholes |
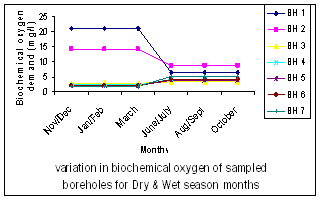 | Figure 6. Variation in Biochemical Oxygen Content |
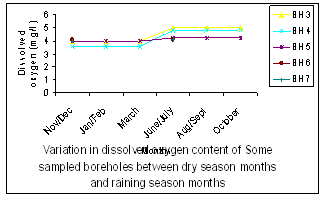 | Figure 7. Variation of Dissolved Oxygen Content |
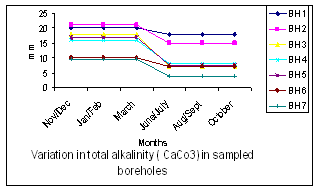 | Figure 8. Variation in Total Alkalinity (CaCo3) in Boreholes Sampled |
|
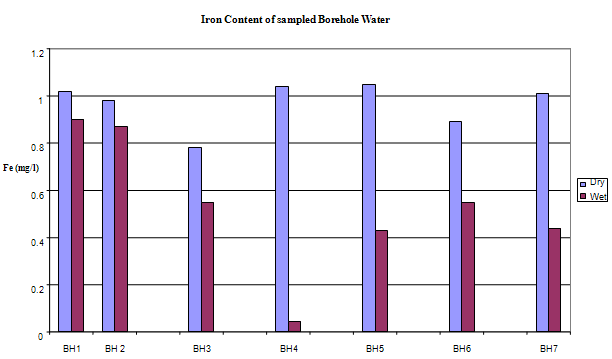 | Figure 9. Iron Concentration in sampled Boreholes |
5. Summary and Conclusions
- The most prevalent ionic concentration includes iron, calcium, magnesium, Sodium, potassium, chloride, and bicarbonate. The trend of occurrence shows the following relationship:
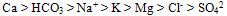 The main naturally occurring iron minerals are magnetite, hematite, goethite and siderite. The Niger Delta region experiences long duration and intensive rainfall couple with high temperature; these weather phenomena accelerate weathering of the geologic formation in the area. The weathering processes release the iron element into waters, under oxygen-poor conditions; it mainly occurs as binary iron. But under acidic and neutral, oxygen-rich conditions it becomes part of many organic and inorganic chelation complexes that are generally water soluble and the dissolved iron is mainly in the form of Fe(OH)2+ (aq).Usually there is a difference between water soluble Fe2+ compounds and water insoluble Fe3+ compounds. The latter are only water soluble in strongly acidic solutions, but water solubility increases when these are reduced to Fe2+ under certain conditions. Iron does not clearly alter in pure water or in dry air, but when both water and oxygen are present (moist air), iron corrodes. Its silvery colour changes to a reddish-brown, because hydrated oxides are formed. Dissolved electrolytes accelerate the reaction mechanism, which is as follows:4Fe + 3O2 + 6H2O -> 4Fe3+ + 12OH- -> 4Fe(OH)3 or 4FeO(OH) + 4H2OUsually the oxide layer does not protect iron from further corrosion, but is removed so more metal oxides can be formed. Electrolytes are mostly iron (II) sulphate, which forms during corrosion by atmospheric SO2. In sea regions atmospheric salt particles may play an important role in this process. Iron (II) hydroxide often precipitates in natural waters giving the characteristic brown colour to the water when the mg/l is highly elevated. The manganese level from the water sample is 0.062625mg/l the WHO accepted limit is 0.05. The slight increase in elevation above the recommended mg/l could be associated with the elevated iron level.The implication of the finding is that groundwater quality improves away from industrial settings, traffic activities. This inference is instructive for water resource developers in Yenagoa and environs. The recent drive towards industrialization and the attendant urbanization means a greater demand for groundwater in the area. New groundwater abstraction schemes are recommended. Future success in understanding the dynamic nature of groundwater systems will rely on continued and expanded data collection at various scales, improved methods for quantifying heterogeneity in subsurface hydraulic properties, enhanced modelling tools and understanding of model uncertainty, and greater understanding of the role of climate and interactions with surface water.
The main naturally occurring iron minerals are magnetite, hematite, goethite and siderite. The Niger Delta region experiences long duration and intensive rainfall couple with high temperature; these weather phenomena accelerate weathering of the geologic formation in the area. The weathering processes release the iron element into waters, under oxygen-poor conditions; it mainly occurs as binary iron. But under acidic and neutral, oxygen-rich conditions it becomes part of many organic and inorganic chelation complexes that are generally water soluble and the dissolved iron is mainly in the form of Fe(OH)2+ (aq).Usually there is a difference between water soluble Fe2+ compounds and water insoluble Fe3+ compounds. The latter are only water soluble in strongly acidic solutions, but water solubility increases when these are reduced to Fe2+ under certain conditions. Iron does not clearly alter in pure water or in dry air, but when both water and oxygen are present (moist air), iron corrodes. Its silvery colour changes to a reddish-brown, because hydrated oxides are formed. Dissolved electrolytes accelerate the reaction mechanism, which is as follows:4Fe + 3O2 + 6H2O -> 4Fe3+ + 12OH- -> 4Fe(OH)3 or 4FeO(OH) + 4H2OUsually the oxide layer does not protect iron from further corrosion, but is removed so more metal oxides can be formed. Electrolytes are mostly iron (II) sulphate, which forms during corrosion by atmospheric SO2. In sea regions atmospheric salt particles may play an important role in this process. Iron (II) hydroxide often precipitates in natural waters giving the characteristic brown colour to the water when the mg/l is highly elevated. The manganese level from the water sample is 0.062625mg/l the WHO accepted limit is 0.05. The slight increase in elevation above the recommended mg/l could be associated with the elevated iron level.The implication of the finding is that groundwater quality improves away from industrial settings, traffic activities. This inference is instructive for water resource developers in Yenagoa and environs. The recent drive towards industrialization and the attendant urbanization means a greater demand for groundwater in the area. New groundwater abstraction schemes are recommended. Future success in understanding the dynamic nature of groundwater systems will rely on continued and expanded data collection at various scales, improved methods for quantifying heterogeneity in subsurface hydraulic properties, enhanced modelling tools and understanding of model uncertainty, and greater understanding of the role of climate and interactions with surface water. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML