-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2012; 2(2): 14-19
doi: 10.5923/j.re.20120202.03
Soil Microarthropods in a Secondary Rainforest, Rivers State, Nigeria - IV- The Impact of Oil Pollution on Their Vertical Distribution
Samuel N. Okiwelu 1, Tambeke N. Gbarakoro 1, Chris O. Umeozor 1, Adetola M Badejo 2
1Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria
2Department of Zoology, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Samuel N. Okiwelu , Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
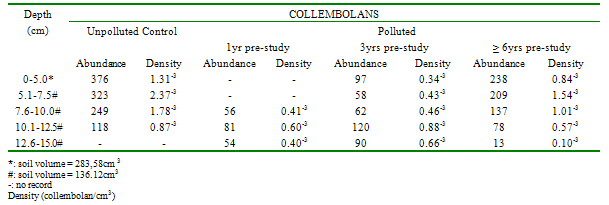
Studies were undertaken, May, 2007-April, 2009, to document species richness, bioindicator species, abundance, density, vertical distribution, etc. of soil microarthropods (mites, collembolans) in unpolluted and polluted habitats in a secondary rainforest, Rivers State, Nigeria. This paper focuses on the effects of oil pollution on the vertical distribution of these mesofauna. Sampling was effected monthly by a bucket-type auger to a depth of 15.0cm in four habitat-types (unpolluted, polluted by oil spills approximately 1yr, 3yrs and 6yrs pre-study). The modified Bukard model of the Berlese-Tullgren funnel was used for extraction. Identifications were undertaken using standard keys and unidentified specimens were compared to type specimens. In the unpolluted and polluted (3 and 6yrs pre-study) habitats, there was an inverse relationship between mite/ collembolan abundance/density (except in the Prostigmata) and soil depth; however, the correlations were not significant. In contrast, there was a significant direct correlation between mite abundance/density and depth in the habitat, polluted 1yr pre-study (F=29.11; df=1.3; p<0.05). In the unpolluted habitat, approximately 80% of all mites and 90% of collembolans were collected within the range 0.00-10.0cm. In the habitat, polluted 1yr pre-study, no mites were collected above a depth of 5.0cm and 70% were found below 10.0cm. In the habitats, polluted 3 and 6yrs pre-study, approximately all mites were found above a depth of 10.0cm. There were direct relationships between collembolan densities and soil depths in the two habitat-types (polluted 1 and 3 yrs pre-study) but the correlations were not significant (F=6.22; df= 1.3; p> 0.05). In the unpolluted habitat, approximately 90% of all collembolans were found above a depth of 10.0cm; this declined to 30% in the habitat, polluted 1yr pre-study, and rose to 50% and 85% in the habitats, polluted 3 and 6yrs pre-study.
Keywords: Soil Microarthropods, Oil Pollution, Effects, Vertical Distribution
Article Outline
1. Introduction
- Globally, investigations have been conducted on the responses of soil microarthropods (mites and collembolans) to various stressors: site preparation techniques[1] heavy metals[2-5], organic carbon content[6-8] organic manure[9], inorganic fertilizer[10], ash treatment of sour, acidic coils[11], herbicides and pesticides[12,6]. The effects of petroleum spill on these microarthropods have received limited attention. Studies on the soil microarthropods of tropical Africa have been minimal, compared to those in the palaeartic and neartic regions. Fortunately,[13-16] have made significant progress in the taxonomy and ecology of soil microarthropods in Nigeria. However, their studies were restricted to a depth of 7.50cm in southwest Nigeria. Some of the results from the current studies have been documented[17,18]; this report focuses on the vertical distribution of soil microarthropods in 4 habitat-types (unpolluted, polluted by an oil spill; 1yr, 3yrs and 6yrs pre-study) in a secondary forest, Rivers State, Nigeria.
2. Materials and Methods
2.1. Study Area
- The habitat-types were located in a secondary lowland rainforest in Tai Local Government Area, Rivers State, Nigeria. Two of the habitats (unpolluted and polluted 3yrs pre-study), 04º44.16N, 007º12.32´E and 04º44.14N, 007º29´ respectively, were at Norkpo; the habitats polluted 1yr and 6yrs pre-study were sited at Kporgior and Gio respectively (Figure 1).
2.2. Methods
- A 3500m2- area was demarcated within each habitat; it was subsequently divided into twelve 24x12m sub-plots to ensure total coverage during monthly sampling over a 2-year period, May, 2007-April, 2009. Soil samples were taken monthly with an 8.5cm-diameter bucket- type auger at the southern section of each sub-plot during the first year, May 2007-April 2008. In the second year, May 2008-May 2009, samples were taken from the northern section. Depths of soil collections were: 0-5.0 cm, 5.1-7.5 cm, 7.6-10.0 cm. 10.1-12.5 cm 1nd 12.6-15.0 cm. To ensure that all the soil in each range was collected, the auger was rotated clockwise and anti-clockwise, until the entire soil was taken. Each sample was placed in a plastic bag, labelled and taken to the laboratory for analyses, by a 3-stage process (extraction, sorting, and identification). The modified Bukard model of the Berlese-Tullgren funnel was used for extraction[19]. The extractor complex consisted of two 8-unit rows, enclosed in a wooden cabinet. Description of the extractor complex and extraction procedure has been documented[20]. The duration of extraction was 7 days. The extracts containing soil microarthropods were placed in Petri-dishes under a dissecting microscope; the mites and collembolans were carefully removed. Temporary slides were prepared and identification undertaken under a compound microscope at the Entomology Research Laboratory, Department of Animal and Environmental Biology, University of Port Harcourt. Identification keys by[21-23] were used. Unidentified specimens were taken to the Laboratory of Systematics and Ecology of Microarthropods, Department of Zoology, Obafemi Awolowo University, Ile-Ife, Nigeria, where they were compared to type specimens.
 | Figure 1. Study Area |
3. Results
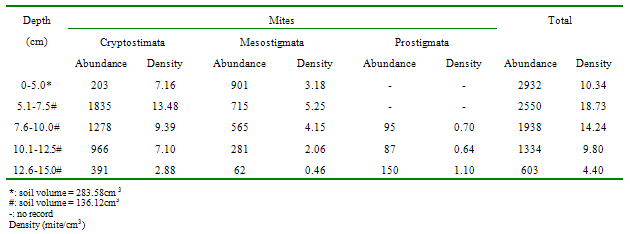
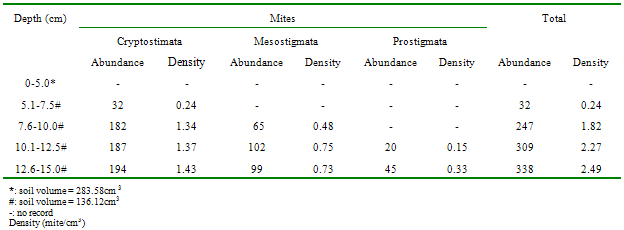
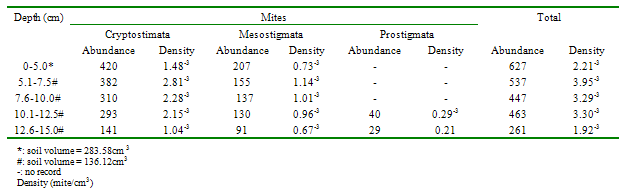
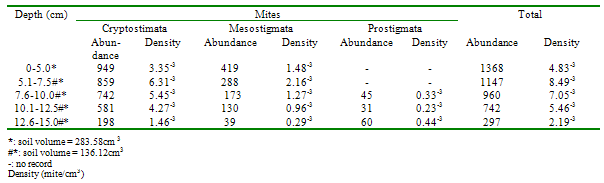
- Approximately 80% of all mites and 90% of collembolans were collected within the range, 0.00-10.0cm in the unpolluted habitat (Table 1). Similar patterns were observed among the Cryptomastigota and Mesostigmata; but in the Prostigmata, there was a significant positive correlation between soil depth and Prostigmata (r=0.937; F=21.54; df=1, 3; P<0.05). No Prostigmata were collected at depths above 7.5cm. In contrast, in the habitat, polluted 1yr pre-study, no mites were collected above 5.0cm; there were significant positive correlations between soil depth and densities of total mites (r=0.952; F=29.11; df=1, 3; P<0.05) (Figure 2), Cryptostigmata (r=0.906; F=13.80; df=1, 3; P<0.05) (Figure 3), Mesostigmata (r=0.936; F=21.12; df=1, 3; P<0.05) (Figure 4), and Prostigmata (r=0.877; F=10.2; df=1, 3; P<0.05) (Figure 5) (Table 2). Approximately 70% of all mites in the habitat polluted (1yr pre-study), were collected below 10.00cm (Table 2).In the habitat polluted 3yrs pre-study, there was an inverse relationship between soil depth and total mite abundance but the correlation between soil depth and density was not significant (F=0.21, df=1,3; P>0.05); similar patterns were observed in Cryptostigmata, Mesostigmata, Prostigmata. Approximately 70% of all mites in this habitat were collected above 10.0cm (Table 3). In the habitat, polluted 6yrs pre-study, there was an inverse relationship between total mite numbers and depth, but the correlation between soil depth and mite densities was not significant (F=1.47, df=1,3 P>0.05); similar patterns were observed between soil depth and Cryptostigmata, Mesostigmata and Prostigmata densities (Table 4). In the unpolluted habitat, approximately 90% of all collembolans were collected above 10.0cm while only 30% of collembolans in the polluted (1yr pre-study) were collected in this range.There was an increase to 50% of collembolans above 10.0cm in the polluted (3yrs pre-study) and 85% in the polluted habitat (6yrs pre-study). There were inverse relationships between soil depths and collembolan abundance in the unpolluted habitat and habitat, polluted 6yrs pre-study but the respective correlations were not significant (F=3.2; df=1, 3; P>0.05; F=3.86, df=1, 3; P>0.05). In the habitats polluted 1yr and 3yrs pre-study, there were direct relationships between soil depths and collembolan densities but the correlations were not significant (F= 6.22. df=1, 3; P>0.05).Analyses of total Hydrocarbon content of soil from the four habitats: unpolluted, polluted 6yrs, 3yrs and 1yr pre-study, showed concentrations of 10.0, 12.5, 237.5 and 680.0mg/kg respectively.
|
|
|
|
|
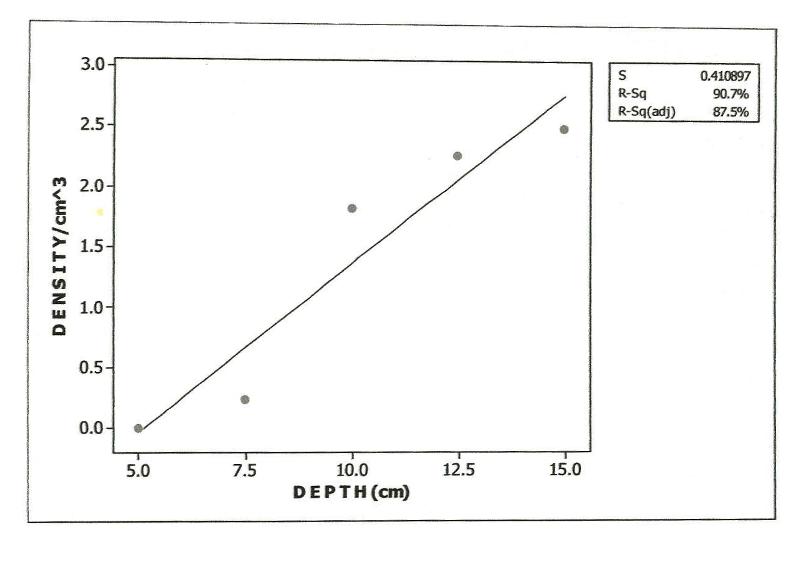 | Figure 2. Relationship of total mite density to soil depth in habitat, polluted 1yr pre-study |
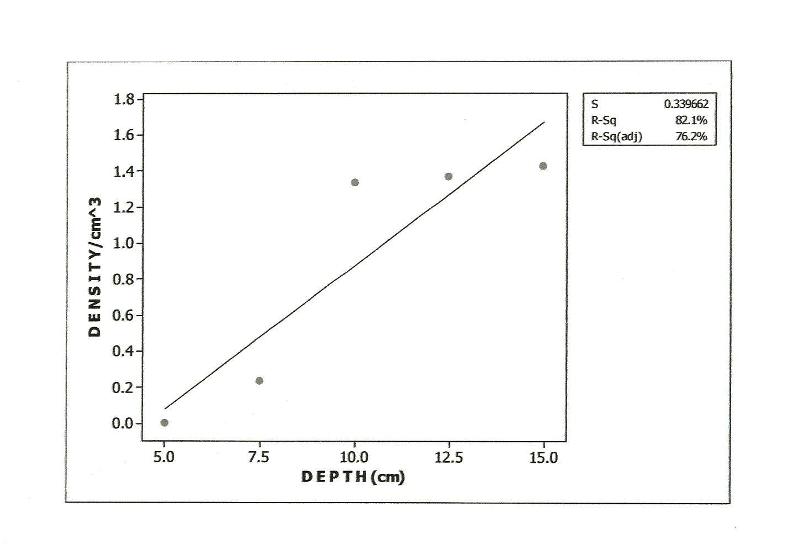 | Figure 3. Relationship between Cryptostigmata densities and soil depth in habitat polluted 1yr pre-study |
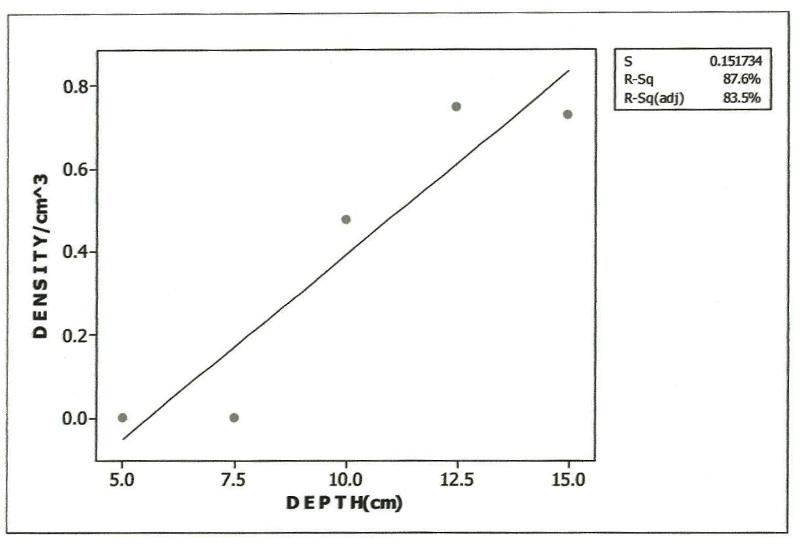 | Figure 4. Relationship between Mesostigmata densities and Soil Depth in Habitat, Polluted 1yr pre-study |
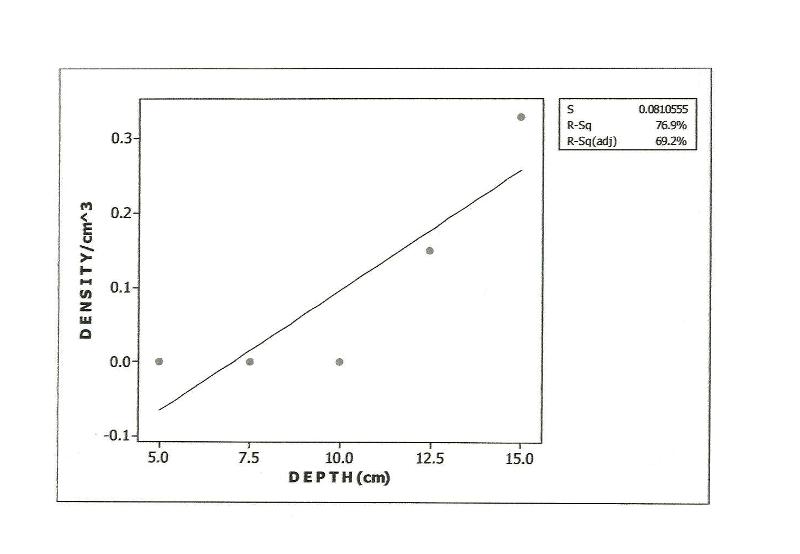 | Figure 5. Relationship between Prostigmata Densities and Soil Depth in Habitat, Polluted 1yr Pre-study |
4. Discussion
- In the unpolluted habitat, 80% of all mites and nearly 80% of all Collembolans were found at depths above 10.0cm. In contrast, the effect of the oil spill in the polluted habitat (1yr pre-study) was the reduction of mites in this zone to 30%; there was also a significant reduction in overall mite numbers. It was apparent that the effects of the oil spill were more pronounced in the uppermost layers, 0-10.0cm. These effects were probably direct (lethal concentrations) and/ or indirect (adversely affecting food sources, microarthropod reproductive rate or soil quality). Seniczak et al. classified Oribatids into 3 categories (quite susceptible, less susceptible, and tolerant) based on their reaction to heavy metals[2]. These categories may also be applicable to their responses to the oil spill. The low- dispersing, near sedentary, low metabolic Oribatids, the mainly predaceous Gamasid (Mesostigmata) and the Prostigmata were all adversely affected in the 0.0-10.0cm range. The low numbers found below 10.0cm were probably species in Seniczak et al. tolerant category[2]. It is apparent that Prostigmata, by predominantly inhabiting depths below 10.0cm may have adopted this strategy for the avoidance of unfavourable conditions[6]. Percents of mites and collembolan species that re-colonized the top 10.0cm of soil rose from 70% (mites) and 50% (collembolans) in the habitat polluted 3yrs pre-study to 75% (mites) and 85% (Collembolans) in the habitat polluted 6yrs pre-study. Although re-colonization of the top 10.0cmof soil based on species richness was noticeable in the habitat polluted 3yrs pre-study, there was no corresponding increase in abundance/density. Abundance/density in the most recently polluted (1yr pre-study) habitat was about 10.0% of that in the unpolluted habitat, 25% in the habitat polluted 3yrs pre-study and 50% in the habitat polluted (6yrs pre-study). The re-colonization (based on abundance/density) was more rapid in collembolans: 17.91% (1yr pre-study), 40.05% (3yrs pre-study) and 63.32% (6yrs pre-study)[24]. This is not surprising because the Oribatids, the dominant mite order, have characteristics that render them unable to respond quickly to short-term hard stresses[25].
5. Conclusions
- In the unpolluted habitat, most of the mites (Cryptostigmata, Mesostigmata) and Collembolans were found in the top 10.0cm-soil where they play a key role in decomposition and mineralization processes. The oil spill reduced abundance and caused movement of the mesofauna to lower layers, particularly in the habitat polluted 1 yr pre-study. Re-colonization of the top 10.0cm-soil occurred in habitats polluted 3yrs and 6yrs pre-study. The Prostigmata occupied lower layers, below 7.5cm in the unpolluted and polluted habitats.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML