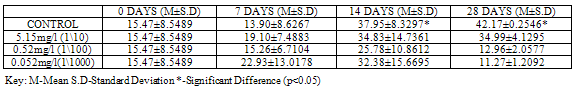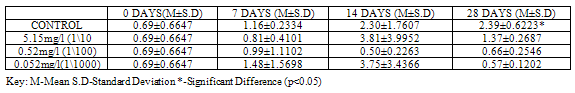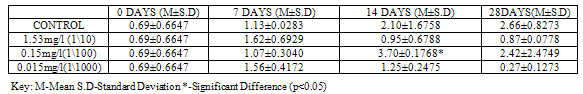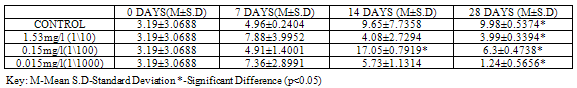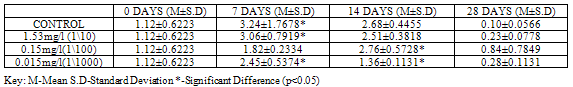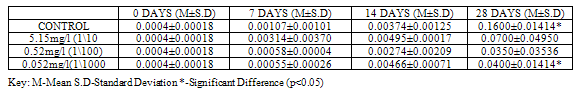Joseph K. Saliu, Kafilat A. Bawa-Allah
Department of Zoology, University of Lagos, Lagos, 23401, Nigeria
Correspondence to: Joseph K. Saliu, Department of Zoology, University of Lagos, Lagos, 23401, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Toxicological effects of sub lethal concentrations of lead and zinc salts (Pb(NO3)2 and ZnCl2) on the antioxidant enzymes of post juvenile Clarias gariepinus in laboratory bioassays was investigated, using the liver. Oxidative stress enzymes and substrates (Glutathione-S-Transferase (GST), Glutathione (GSH), Superoxide dismutase (SOD), and Catalase (CAT) ) showed irregular activities during the first two weeks of the experiment, but a significant variation (p<0.05) from levels detected in controls after 28 days in fishes exposed to both metals. Increased levels of GST-GSH and reduced levels of SOD and CAT occurred in fishes exposed to ZnCl2 as compared to control while there were no variations in the levels of Malondialdehyde (MDA). In fishes exposed to Pb(NO3)2 however, GST-GSH, SOD, CAT and MDA all reduced when compared to control. There is need for more research on the oxidative defense response of Clarias gariepinus to persistent levels of high concentrations of heavy metals .There is also a need for comparison of existing field and laboratory studies so as to fully exploit the bio markers of oxidative stress as indicators of environmental pollution in tropical fresh water systems.
Keywords:
Antioxidant Enzymes, Oxidative Stress, Lead, Zinc, Clarias gariepinus
Cite this paper: Joseph K. Saliu, Kafilat A. Bawa-Allah, Toxicological Effects of Lead and Zinc on the Antioxidant Enzyme Activities of Post Juvenile Clarias gariepinus, Resources and Environment, Vol. 2 No. 1, 2012, pp. 21-26. doi: 10.5923/j.re.20120201.03.
1. Introduction
Oxidative stress, a pathological process relating to overproduction of reactive oxygen species (ROS) in tissues is one important general toxicity mechanism for many xenobiotics. Oxidative stress was shown to be induced by anthropogenic contaminants such as persistent organic pollutants (POPs), heavy metals, and also by toxins produced during massive blooms of cyanobacteria[5,13]. Many organisms including fishes have evolved mechanisms to counteract the impact of ROS. These include various antioxidant defense enzymes such as superoxide dimutases, catalase and glutathione S- transferase.Biomarkers of oxidative stress have been used to study the biochemical changes brought about by heavy metal poisoning in aquatic organisms in the last decade[9] studied biochemical changes induced by heavy metal pollution in marine fishes at khomse coast, Libya and found out that superoxide dismutase (SOD) and catalase (CAT) activities in liver tissues of three collected fishes showed difference in enzyme activity parallel to metal bioaccumulation. They also noted that lipid peroxidation levels indicated by increase in malonyldialdehyde (MDA) were parallel to metal bioaccumulation[8] evaluated the levels of heavy metals and certain biomarkers of oxidative stress as surrogate bioindicatorsof aquatic pollution in Clarias gariepinus. They found that Zn, Cd, Pb, and Cu were accumulated in high concentrations in the liver, kidney, heart and gills of the fish from Ogun river and they also found that activities of superoxide dismutase (SOD), gluthathione s transferase (GST) and glutathione (GSH) were elevated in all the organs except the gills and concluded that the significant increase in these organs may be a response to oxidative stress induced by the presence of heavy metals.The African catfish, Clarias gariepinus is commonly cultured in fish farms in Nigeria and is a common fresh water fish widely consumed locally.This study aims to determine the level of oxidative enzyme response in post juveniles of C. gariepinus after exposure to sublethal concentrations of zinc and lead.
2. Materials and Method
2.1. Test Animals; Source and Acclimatization
C.gariepinus post juveniles (6-8 weeks old) were purchased from local fish farms in Surulere Lagos and transported in polythene bags half filled with pond water to holding tanks (length: 45.00cm, height: 34.00cm, bottom diameter: 25.00cm and top diameter:35.00cm) in the laboratory. The post juveniles were kept in the plastic holding tanks containing dechlorinated water, to acclimatize to laboratory conditions (28 ± 20℃, R.H 70 ± 2%) for a period of seven days before they were used in the bioassays. The post juveniles were fed with fish food (Coppens,) at 3% of body weight twice daily, and the water was changed once every 48hours, aerating it continuously with Bzagdon air pump (double type 1200).
2.2. Test Compounds
Zinc as ZnCl2.4H2O analar grade (molecular weight 136.28 g, purity 98%) and Lead as Pb(NO3)2 (molecular weight 331.21 g, purity 99.5%), manufactured by J.T. Baker, a division of Mallinekrodt Baker Inc.
2.3. General Bioassay Techniques
2.3.1. Bioassay Containers
Circular plastic bowls (volume: 6 liters, bottom diameter: 22 cm and top diameter: 33 cm) were used as bioassay container.
2.3.2. Preparation of Test Media
To prepare test media for bioassays, computed volume of stock solutions was pipetted out and made up to 5 litres. Preliminary studies showed that 5 post juveniles survived well in 5 litres of media for 7 days without aeration.
2.3.3. Selection of Animal for Bioassay
Active post juveniles of similar age and size (age: 6-8 weeks old, mean snout to tail length: 15.00-22.00cm, mean weight: 31.00-55.00g) were taken from holding tanks and randomly assigned to experimental containers.
2.4. Biochemical Studies of Clarias gariepinus Exposed to Sublethal Concentrations of (Zn and Pb) acting singly in Semi Static Bioassays
8 active post juveniles of similar age and size were exposed to sub lethal concentration and untreated control in 2 replicates (4 post juveniles per replicate). These series of bioassays went on for 28 days and the semi static bioassay procedure was adopted in order to avoid drastic changes in concentration of test media via evaporation and excessive reduction in dissolved oxygen level. In the semi static procedure, each test media was changed into a fresh solution of exactly the same concentration of heavy metal salt or untreated control respectively once every four days, transferring the same exposed test animals into the freshly prepared test media over the 28 day period of the experiment.At time intervals of 7, 14 and 28 days, one live C. gariepinus per replicate, (making two per treatment and two from untreated control )were randomly selected , dissected and the liver kept refrigerated pending further analysis. C. gariepinus were exposed to sublethal concentrations of test heavy metals in separate experiments as follows:a) ZnCl2.4H2O was tested at: 1.53mg/l (0.1 of 96hrLC50), 0.15mg/l (0.01 of 96hrLC50), 0.015mg/l (0.001 of 96hrLC50) b) Pb(NO3)2 was tested at 5.15mg/l (0.1 of 96hrLC50), 0.52mg/l (0.01 of 96hrLC50), 0.052mg/l (0.001 of 96hrLC50)
2.5. Biochemical Studies of Liver of Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Pb(NO3)2
The following substrate and enzyme activity were analyzed in the liver of Clarias gariepinus exposed to sublethal concentrations of ZnCl2 and Pb(NO3)2.
2.5.1. Catalase Activity
Catalase activity was determined by measuring the decrease in absorbance at 240nm due to the decomposition of H2O2 in a UV recording spectrophotometer. The reaction mixture (3ml) contained 0.1ml of tissue homogenate in phosphate buffer (50mM, pH 7.0) and 2.9ml of 30mM H2O2 in phosphate buffer pH 7.0. An extinction coefficient for H2O2 at 240nm of 40.0 M-1cm-1was used for the calculation [1]. The specific activity of catalase was expressed as moles of H2O2 reduced per minute per mg protein.
2.5.2. Superoxide Dismutase (SOD) Activity
Superoxide dismutase activity was determined by its ability to inhibit auto-oxidation of epinephrine which was estimated by the increase in absorbance at 480nm as described by[12]. The reaction mixture (3ml) contained 2.95ml 0.05 M sodium carbonate buffer pH 10.2, 0.02ml of tissue homogenate and 0.03ml of epinephrine, 0.005 N HCL was used to initiate the reaction. The reference cuvette contained 2.95ml buffer, 0.03ml of substrate (epinephrine) and 0.02ml of water. Enzyme activity was calculated by measuring the change in absorbance at 480nm for 5 minutes.
2.5.3. Glutathione-S-Transferase (GST) Activity
The assay is based on the fact that all GST demonstrate a relatively high activity with 1-chlor-2, 4-dinitrobenzene as the second substrate. Consequently, the conventional assay for GST activity utilizes 1-chloro-2, 4-dinitrobenzene as substrate. The subsequent conjugation of this substance with reduced glutathione, results in a shift of its absorption maximum to a longer wavelength. The absorption increase at the new wavelength of 340nm provides a direct measurement of enzymatic reaction.Reagents: 1-Chloro-2, 4-Dinitrobenzene (20mM); 3.37mg of 1-chloro-2, 4-dinitrobenzene (CDNB) was dissolved in 1ml of ethanol; reduced Glutathione (0.1M), 30.73mg of reduced glutathione was dissolved in 1ml of 0.1M phosphate buffer (pH 6.5); 0.1M Phosphate Buffer (pH 6.5), this was prepared by dissolving 4.96g of dipotassium hydrogen phosphate and 9.73g of potassium dihydrogen phosphate in little amount of distilled water and then made up to the mark in a 1litre volumetric flask. The pH was adjusted to 6.5.Procedure: The medium for the estimation was prepared as shown below and was allowed to run for 60 seconds each time before the absorbance was read against the blank at 340nm. The temperature was maintained at approximately 310C. The absorbance was measured using a spectrophotometer Assay Medium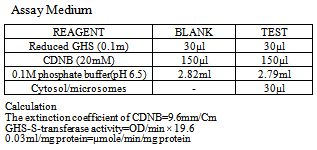
2.5.4. Glutathione (GSH)
The reduced glutathione content of the tissue as non protein was estimated according to the method described by[11]. To the tissue homogenate, 10% TCA was added, centrifuged. 1.0ml of supernatant was treated with 0.5ml of Ellman’s reagent (19.8mg of 5, 5-dithiobisnitro benzoic acid (DTNB) in 100ml of 0.1% sodium nitrate) and 3.0ml of phosphate buffer (0.2M, pH 8.0). The absorbance was read at 412nm.
2.5.5. Lipid Peroxidation
Malondialdehyde (MDA) an index of lipid peroxidation was determined using the method of [4].1.0ml of the supernatant was added to 2ml of (1:1:1 ratio) TCA-TBA HCL reagent (thioarbituric acid 0.37%, 0.24N HCL and 15% TCA) tricarboxylic acid-thioarbituric acid-hydrochloric acid reagent boiled at 100℃ for 15 minutes, and allowed to cool. Flocculent materials were removed by centrifuging at 3000 rpm for 10 minutes. The supernatant was removed and the absorbance read at 532nm against a blank. MDA was calculated using the molar extinction coefficient for MDATBA- complex of 1.56×105M/Cm.
2.6. Statistics
One-way analysis of variance (ANOVA) was used to compare the means of results obtained. from biochemical analysis, and where a significant difference (p<0.05) was obtained, Duncan test was used to detect the source of the difference.Means of parameters obtained during the period of the experiment for each treated group and control were compared, and those obtained on each sampling day for treated groups and control was also compared.
3. Results
3.1. Biochemical Studies of Liver of Clarias gariepinus Exposed to Sublethal Concentrations of Pb(NO3)2 and Zncl2 Respectively
The biomarkers of oxidative stress analyzed showed significant variations (p<0.05) but no uniform trend at 7 and 14 days, when control and all groups exposed to sub lethal concentrations of the respective heavy metals were compared. However, significant variations (p<0.05) and a uniform trend was observed for the respective biomarkers at 28 days among control and exposed groups to the two heavy metals.
3.1.1. Glutathione-S-Transferase (GST)
GST activity was significantly higher (p<0.05) in control (42.17 ± 0.2546) when compared to groups exposed to sub lethal concentrations of Pb(NO3)2 (34.99±4.1295, 12.96 ± 2.0577 and 11.27 ± 1.2092 ) at 28 days, (Table 1).For test animals exposed to concentrations of ZnCl2, GST activity was lower in control (1.10±0.3748) as compared to the exposed groups (3.13±1.1314, 7.10±0.6152 and 3.91±1.5274 ) at 28 days, with higher values (p<0.05) in group exposed to 1/100 96 hr LC50. (Table2).
3.1.2. Superoxide Dismutase (SOD)
SOD activity was significantly higher (p<0.05) in control (2.39±0.6223) than in groups exposed to concentrations of Pb(NO3)2 (1.37±0.2687, 0.66±0.2546 and 0.57±0.1202) at 28 days (Table 3). In the test animals exposed to ZnCl2, SOD activity was higher (2.66±0.8273), though not significant at (p<0.05) in control when compared to exposed groups (0.87±0.0778, 2.42±2.4749 and 0.27±0.1273) at 28 days, although significant differences (p<0.05) were recorded on other days (Table 4).
3.1.3. Catalase (CAT)
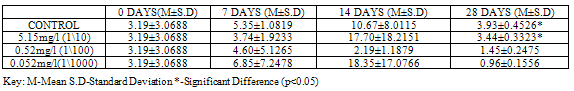
At 28 days, CAT showed the highest activity (p<0.05) with a value of 3.93±0.4526 in control when compared to the test animals exposed to concentrations of Pb(NO3)2 (3.44±0.3323, 1.45±0.2475 and 0.96±0.1556). No significant variations were recorded at earlier sampling days (Table 5).For test animals exposed to concentrations of ZnCl2, CAT activity showed significant variations (p<0.05) at 28 days in all groups, with higher values 9.98±0.5374 recorded in control. (Table 6).
3.1.4. Glutathione (GSH)
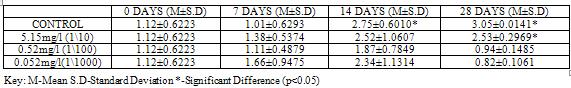
GSH level was higher (p<0.05) in control with a value of 3.05±0.0141, when compared to groups exposed to concentrations of Pb(NO3)2 (2.53±0.2969, 0.94±0.1485 and 0.82±0.1061), at 28 days (Table 7).For test organisms exposed to concentrations of ZnCl2, GSH level was lower (0.10±0.0566) though not significant at (p<0.05) in control at 28 days, (Table 8).
3.1.5. Lipid Peroxidation
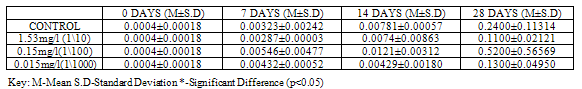
Lipid peroxidation is indicated by the presence of Malondialdehyde (MDA) in tissues. MDA level was higher (p<0.05) in control with a value of 0.1600±0.01414 when compared to groups exposed to concentrations of Pb(NO3)2 (0.0700±0.04950, 0.0350±0.03536 and 0.0400±0.01414) at 28 days (Table 9). MDA level in test organisms exposed to concentrations of ZnCl2 did not vary significantly (p<0.05) when compared to control at 28 days and even at earlier sampling days (Table 10).Table 1. GST activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations Of Pb(NO3)2 and Control
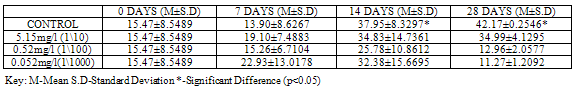 |
| |
|
Table 2. GST activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Control
 |
| |
|
Table 3. SOD activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations of Pb(NO3)2 and Control
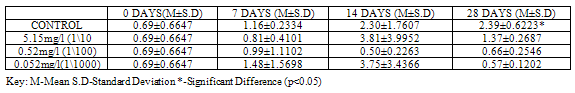 |
| |
|
Table 4. SOD activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Control
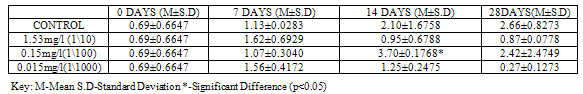 |
| |
|
Table 5. CAT activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations of Pb(NO3)2 and Control
 |
| |
|
Table 6. CAT Activity (U/mg protein) in Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Control
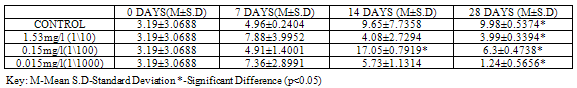 |
| |
|
Table 7. GSH (µmol/L/g wet weight) in Clarias gariepinus Exposed to Sublethal Concentrations of Pb(NO3)2 and Control
 |
| |
|
Table 8. GSH (µmol/L/g wet weight) in Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Control
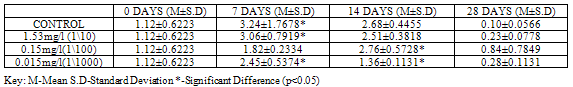 |
| |
|
Table 9. MDA (µmol/L/g wet weight) in Clarias gariepinus Exposed to Sublethal Concentrations of Pb(NO3)2 and Control
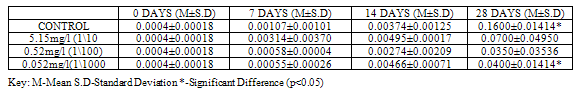 |
| |
|
Table 10. MDA (µmol/L/g wet weight) in Clarias gariepinus Exposed to Sublethal Concentrations of ZnCl2 and Control
 |
| |
|
4. Discussion and Conclusions
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and the cell’s ability to reduce ROS, detoxify reactive intermediates and repair damage that may occur in cellular molecules. This imbalance may occur as a result of increased ROS production, a decrease in defense mechanisms or both. ROS are produced endogenously within the cell, however many environmental parameters including exposure to heavy metals are known to induce oxidative stress[2]. Major biomarkers of oxidative stress include changes in antioxidant enzyme activity and accumulation of oxidative damage products as indicated by post juvenile Clarias gariepinus exposed to lead and zinc in this study. Glutathione S-transferase (GST) in conjunction with glutathione (GSH) detoxifies lipid hydroperoxides from the system. Results from this study showed that GST and GSH reduced after 28 days, in post juveniles of C. gariepinus exposed to concentrations of Pb(NO3)2 when compared to the control, while GST and GSH increased after 28 days in organisms exposed to ZnCl2 when compared to the control. The reduced activity observed for organism exposed to Pb(NO3)2 is in agreement with[10] who studied Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons and reported decreased level of GST and GSH in the exposed fishes as compared to the control. Increased activity observed in organisms exposed to ZnCl2 is also in line with work done by[7] who studied the biomarkers of oxidative stress and heavy metal levels including zinc as indicators of environmental pollution in African cat fish from Ogun River. They reported an increase in activity of GST and levels of GSH in liver of catfish from Ogun River as compared to their control site and attributed this to adaptive and protective role of the biomolecule against oxidative stress induced by heavy metals.Superoxide dismutase (SOD) converts superoxide radicals (O2-) generated in peroxisomes and mitochondria to hydrogen peroxide, catalase (CAT) then acts on the hydrogen peroxide converting them to harmless molecules. Results from this study showed a relatively similar trend in the activities of SOD and CAT in the two groups of organisms exposed to sub lethal concentrations of ZnCl2 and Pb(NO3)2 .SOD and CAT reduced after 28 days in organisms exposed to concentrations of Pb(NO3)2 and ZnCl2 when compared to their respective control. This finding is also in line with a previous investigation made by[10] in which Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus were useful as biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. He also reported a decrease in activities of SOD and CAT of exposed fishes and attributed this to the SOD-CAT system, stating that an inhibition of the enzyme SOD will expectedly result in a reduction in the activity of the enzyme CAT, due to a decrease in H2O2 generation from SOD activities.Malondialdehyde (MDA) is one of the oxidative damage products of lipid peroxidation. Its presence in tissues indicates oxidative stress. Results from this study showed that MDA reduced after 28 days in organisms exposed to concentrations of Pb(NO3)2 when compared to the control. For organisms exposed to concentrations of ZnCl2, there were no variations in level of MDA at 28 days when exposed groups were compared to the control. Conversely[6] who studied biomarkers of oxidative stress and heavy metal (including lead and zinc) levels as indicators of environmental pollution in some selected fishes in Lagos, Nigeria, reported increased levels of MDA in liver of fishes caught from polluted sites as compared to control sites and attributed this to the pollution of the test site. This difference can be attributed to the fact that[6] conducted a field survey, while this study was laboratory based. Oxidative stress due to the toxic effect of pollutants is usually indicated by increased levels of products of oxidative damage (MDA) and subsequent increase in defense enzymes (GST-GSH, SOD CAT) in response to the stress [6-field observations] or decrease due to overwhelming effect of the pollutants ([10,7]-laboratory data). However, results from this study did not follow either of these trends. In test organisms exposed to concentrations of Pb(NO3)2, oxidative enzymes (GST-GSH, SOD CAT) reduced with concurrent reduction in product of oxidative damage (MDA) as compared to control. This undefined trend can be attributed to the fact that toxicity and bioavailability of heavy metals can be affected by physico chemical parameters of the prevailing medium. A few workers have commented on the effect of water hardness on toxicity and bioavailability of lead salts[3] studied The Preservative effects of Quanats Water to reduce Lead Acetate Toxicity On Capoeta fusca. They observed that lead acetate in soft water (10mg/l) was highly toxic to the fish, but in hard water (340mg/l) it had little toxicity. Water hardness of test media used in this study ranged from 40mg/l to 130mg/l and this can be a possible explanation of the low toxicity, variable bioaccumulation and undefined enzymatic defense response of fishes exposed to the metal.For the test organisms exposed to ZnCl2, SOD-CAT reduced while GST-GSH increased with no change in MDA as at 28 days when compared to the control. This trend can be related to the trend of bioaccumulation of the heavy metal where rate of bioaccumulation reduced as the experiment progressed, with lowest values at 28 days. The increased activity of the GST-GSH defense system helped the organisms to regulate bioaccumulation of the heavy metals to levels the body can tolerate and overcome oxidative stress as indicated by the level of MDA. This may not be unrelated to the fact that zinc is an essential heavy metal that is useful in minute quantities to the body.The uncommon trends of oxidative defense systems observed in this study justifies the need for more research, especially to study environmental factors that affect the oxidative defense response of organisms to persistent levels of high concentrations of heavy metals in their environment. There is also need for extensive evaluation and comparison of data obtained from field studies and those obtained from laboratory studies. There are some environmental factors that are at play in the field which cannot be replicated under laboratory conditions, and which may also have significant effects on oxidative defense response of organisms to persistent levels of high concentrations of heavy metals. Evalu- ation of field observations viz laboratory data will also help to establish the usefulness of biomarkers as indicators of environmental pollution.
References
| [1] | Aebi, H. (1984). Catalase in vitro. In: Colowick SP,Kaplane NO eds. Methods in Enzymol. 105: 121-126 |
| [2] | Almroth, B.C.(2008).Oxidative Damage in Fish used as Biomarkers in Field and Laboratory Studies. Department of Zoology/Zoophysilogy Goteborg University Sweden. 74pp |
| [3] | Arash, O.Sohrab, M. and Homayoon F.(2009). Preservative Effects of Quanats Water to Reduce Lead Toxicity on Capoeta fusca .Journal of Fisheries and Aquatic science. 4(1): 50-56 |
| [4] | Buege, J.A. and Aust, S.D. (1978).Microsomal Lipid Peroxidation. Methods Enzymol. 52: 302-310 |
| [5] | Ding, W. X., Shen, H.M., Shen, Y., Zhu, H.G., Ong, C.N., (1998). Microcystic Cyanobacteria causes Mitochondrial Membrane Potential Alteration and Reactive Oxygen Species Formation in Primary Cultured Rat Hepatocytes. Environmental Health Perspective. 106: 409-413 |
| [6] | Doherty, V.F.,Ogunkuade, O.O. and Kanife, U.C.(2010). Biomarkers of Oxidative Stress and Heavy Metal Levels as indicators of Environmental Pollution in Some Selected Fishes in Lagos, Nigeria. American-Eurasian Journal of Agriculture & Environmental Science. 7 (3): 359-365 |
| [7] | Faramobi, E.O., Adewole, O.A., and Ajimoko, Y.R. (2007a). Biomarkers of Oxidative Stress and Heavy Metal Levels as Indicators of Environmental Pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. International Journal of Environmental Research and Public Health. 4(2):158-165 |
| [8] | Faramobi, E.O., Adewole, O.A., and Ajimoko, Y.R.(2007b). Effect of Butachlor on Antioxidant Enzyme Status and Lipid Peroxidation in Fresh Water African Catfish, (Clarias gariepinus). International Journal of Environmental Research and Public Health. 5(5): 423-427 |
| [9] | Metwally, M.A.A., and Fouad, I.M. (2008). Biochemical Changes induced by Heavy Metal Pollution in Marine Fishes at Khomse Coast, Libya. Global Veterinaria 2(6):308-311 |
| [10] | Olagoke, O. (2008). Lipid Peroxidation and Antioxidant Defense Enzymes in Clarias gariepinus as Useful Biomarkers for Monitoring Exposure to Polycyclic Aromatic Hydrocarbons. MSc Theses, University of Lagos, Lagos, Nigeria.70pp |
| [11] | Sedlak, J. and Lindsay, R.H.(1968).Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Analytical Biochemistry. 25: 1192-1205 |
| [12] | Sun, M. and Zigma,S.(1978).An ImprovedSpectrophotometer Assay of Superoxide Dismutase Based On Epinephrine Antioxidation. Analalytic Biochemistry. 90: 81-89 |
| [13] | Van Der Oost, R., Beyer, J., Vermeulen, N. (2003). Fish Bioaccumulation and Biomarkers in Environmental Risk Assessment: A Review. Environmental Toxicolology Pharmacology. 13: 57-149 |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML