Jack Dekker 1, 2, Heather MacKenzie 2, Kevin Chandler 2
1Weed Biology Laboratory, Department of Agronomy, Iowa State University, Ames, Iowa, 50011, USA
2Department of Crop Science, University of Guelph, Guelph, Ontario, N1G 2W1, Canada
Correspondence to: Jack Dekker , Weed Biology Laboratory, Department of Agronomy, Iowa State University, Ames, Iowa, 50011, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The perennial, graminaceous, plant quackgrass (Elymus repens (L.) Gould) is a serious weed problem. When corn is grown continuously, the high amounts of nitrogen fertilizer applied can alter the pH of the soil, and this decrease in soil pH with time may change the weed spectrum present in corn fields. Studies were conducted to determine the effect of four different pH soils (3.7, 4.3, 5.5, 6.2) on the growth of quackgrass rhizome fragments in terms of biomass accumulation and tissue nutrient content. As the soil pH decreased from 6.2 to 3.7, quackgrass plants accumulated less shoot, rhizome and root biomass, as well as less shoot height and numbers of main axis shoots, leaves and rhizome buds. This inhibitory effect of soil pH on quackgrass growth was most apparent in the later six weeks of development, until seedhead anthesis was apparent. In the first four weeks after planting the rhizomes, the reductions in quackgrass growth were best indicated by numbers of leaves and main axis shoots, as well as by shoot height. This reduction in growth associated with lower pH soils probably was due to two mechanisms. The first mechanism could be interference with uptake and incorporation of magnesium and phosphorus into both above and below ground plant parts, as well as with copper and calcium in shoots, and zinc in rhizome and root tissue. A second mechanism could be due to toxicity caused by excessive amounts of manganese in all plant parts, as well as excessive boron in shoot plant parts.
Keywords:
Quackgrass, Agropyron Repens, Elytria Repens, Rhizomes, Nutrient Toxicity, Nutrient Deficiency
Cite this paper:
Jack Dekker , Heather MacKenzie , Kevin Chandler , "The Effects of Soil pH on Elymus Repens Growth and Tissue Nutrients", Resources and Environment, Vol. 1 No. 1, 2011, pp. 20-25. doi: 10.5923/j.re.20110101.03.
1. Introduction
The perennial, graminaceous, plant quackgrass, Elymus repens (L.) Gould (Agropyron repens (L.) Beauv.), is a serious weed problem for many corn growers in southwestern Ontario, as well as many other agricultural areas. When corn is grown continuously, the high amounts of nitrogen fertilizer applied can alter the pH of the soil. A significant decrease in soil pH with time may change the weed spectrum present in corn fields.Although quackgrass has been found growing over a wide soil pH range (Werner and Rioux, 1968), it has been reported to be a basiphilic species (Dale et al., 1965; LeFevre, 1956; Werner and Rioux, 1968). If growth of quackgrass is restricted in acidic soils, this could imply that continuous corn production with high nitrogen fertilization can lead to a reduction in weed competitiveness.With these observations in mind, studies were conducted to determine a) the effect of four different pH soils (3.7, 4.3, 5.5, 6.2) on the growth of the perennial weed quackgrass in terms of biomass accumulation and tissue nutrient content.This is the second article in a series of two on the effects of soil pH on weed growth. The first article evaluated the effects of soil pH on annual weed growth, and on the efficacy of postemergence herbicides on those weeds (Dekker et al., 2006). The second article herein deals with the effects of soil pH on the growth of quackgrass.
2. Methods and Materials
2.1. Soils
Soil was collected from four locations in southwestern Ontario in the spring. At the first location (Norfolk County, Middleton Township, Conc. II, Lot 24) a Watrin Fine Sand was collected with an initial soil pH of 3.7. The field had a history of continuous corn cropping. A Fox Loamy Sand with an initial pH of 4.3 was collected from the second location (Norfolk County, North Walsingham Township, Conc. XIII, Lot 23). Winter wheat was being grown at the time of collection. In the previous year barley was grown, and in the years preceeding that continuous corn. At a third location (Kent County, Howard Township, Conc. IX, Lot 11) a Fox Very Fine Sandy Loam with an initial pH of 5.5 was collected. The last collection site (Oxford County, South Norwich Township, Conc. XI, Lot 10), a corn field, was used to obtain a Fox Loamy Sand with an initial pH of 6.2.Soil pH was determined at the beginning of the experiment from the bulk containers and then again from the plant pots after each of the five harvests. For pH testing, the paste method, using deinonized water and a Corning pH meter and electrodes, was used. All soil tests were conducted by the Ontario Ministry of Agriculture and Food (OMAF) Soil Testing Laboratory, operated by the University of Guelph Department of Land Resource Science, Ontario Agricultural College, Guelph, Ontario.
2.2. Plant Material
Quackgrass rhizomes were collected from the Elora Research Station (Wellington County, Pilkington Township, Conc. I, Lot 8. The soil these rhizomes were growing in was a London Loam with a pH of 7.4. The collected rhizomes were cut into branched six-bud (run 1), or 5-7 bud (run 2), segments and were planted in 10.5 by 15.0 by 10.5cm pots (1654cm2 volume) in run 1 (run 2: 16 by 21 by 25cm pots with a 8400cm2 volume) approximately 2.5cm below the soil surface such that one tip of the rhizome was exposed. The average fresh weight of the rhizome segments used was 0.65g (run 1), or 0.40-0.60g (run 2), at the time of planting. Five pts of quackgrass were used in each of five harvests, resulting in 25 pots per soil type. The plants were watered as needed with water from the City of Guelph system (run 1), or with deionized water (run 2). In run 1 the excess water was allowed to drain freely from the pots, while in run two the pots had plastic liners and no drainage occurred. Fertilization in run 1 was done weekly with 142ml of 20% N-20% P-20% K liquid fertilizer (each application containing 3.00 x 10-3 moles nitrogen (N), 3.87 x 10-3 moles phosphorus (P) as P2O5, and 2.15 x 10-3 moles potassium (K) as K2O). Fertilization in run 2 was done weekly with 315 ml of 20% N-20% P-20% K liquid fertilizer (each application containing 6.85 x 10-3 moles N, 8.85 x 10-3 moles P as P2O5, and 4.91 x 10-3 moles K as K2O). Harvests were conducted every two weeks post planting. The experiment ran for 10 weeks. At each harvest the following data was collected: height of the tallest shoot with leaf extended, number of main axis shoots excluding tillers, total number of tillers per pot, total leaf number per pot, total shoot and rhizome fresh and dry weights, rhizome bud number, stage of development of each shoot system, and a soil sample for each soil type (composite from each pot of the five replications.
2.3. Growth Conditions
Plants were grown in large, walk-in, controlled environmental chambers in the Crop Science Building, University of Guelph. The environmental conditions there were 25-20℃ day-night temperatures, 60% relative air humidity, and a 16h photoperiod with a constant photon flux density of ca. 440 µmol quanta m-2 s-1.Experimental Design. The experiment employed a randomized compete block design (RCBD) with treatments consisting of quackgrass grown in 4 different pH soils. The experiment was conducted twice, with both run 1 and run 2 having 5 replications.
2.4. Plant Nutrient Analysis
Separate shoot and root plus rhizome tissue analyses were conducted on each treatment after all five harvests. Tissue samples from all five replications for each individual soil pH treatment were combined after each harvest. The data on tissue nutrients was analyzed using each of the five harvest dates as a single replication. Since the results from the growth analyses from runs 1 and 2 did not differ, the results from the shoot and rhizome tissue analyses are discussed as representative of the entire experiment. The quackgrass nutrient tissue analyses were conducted by the Plant Analysis Laboratory of the OMAF Soil Testing Laboratory, Department of Land Resource Science, University of Guelph, Guelph, Ontario. All plant material was dried to a constant weight and then ground in a 20-mesh mill fitted with stainless steel knives and screen. The ground material was then placed in moisture-proof containers. Analysis for N, P, K, Ca and Mg proceeded with wet ashing via the procedure of Thomas et al., 1967. For Mn, Cu, Zn, B, Fe and Al analyses dry ashing was done by placing the plant material in a muffle furnace at 470℃ overnight and then disolving it in 0.4 N HCl. After ashing, readings for Al, Ca, Cu, Fe, K, Mg, Mn and Zn were made with a Varian Model AA-175 atomic absorption machine; whereas readings for B, N and P were made with a Technicon Auto Analyzer II.
3. Results
3.1. Effect of Soil pH on Quackgrass Growth
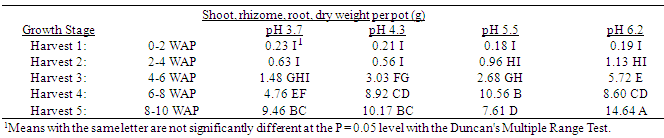
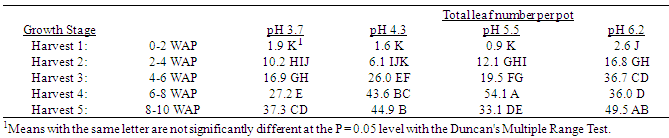
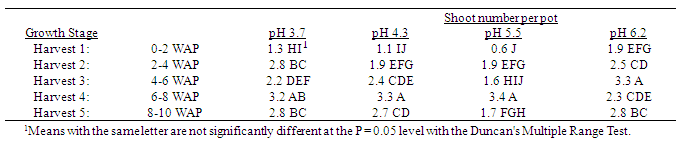
Generally, as the soil pH increased both shoot and rhizome growth of quackgrass decreased. No differences in shoot fresh or dry weight, rhizome plus root fresh or dry matter, or total shoot, rhizome and root fresh or dry weight, or rhizome bud numbers were observed in the first four weeks after planting (Tables 1-7). Generally, after the fourth week of growth, as the soil pH decreased shoot and rhizome biomass, and rhizome bud numbers, decreased. This pattern was most consistently observed, from the fourth week after planting until seedhead anthesis, with shoot dry weight (Table 2), rhizome plus root fresh (Table 3) and dry weight (Table 4), shoot, rhizome and root dry matter (Table 6), and rhizome bud number (Table 7).The effect of soil pH was apparent in the first four weeks as indicated by growth of leaf numbers (Table 8), shoot main axis numbers (Table 9) and shoot height (Table 10). For the 10 week life of the quackgrass plants observed, in most instances leaf and shoot numbers, as well as shoot height, in plants grown at soil pH 6.2 were greater than in those plants grown at pH 4.3 or 3.7. The change in these parameters was not always consistent with consistent changes in soil pH, but did provide indications of the inhibitory effects of lower soil pH on quackgrass growth early in its development. The most consistent indicator of the inhibitory effects of low soil pH on quackgrass growth was leaf number (Table 8), and could serve as the most sensitive early indicator of soil pH stress.
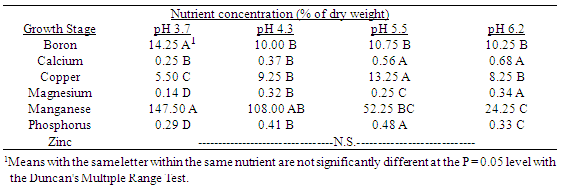
3.2. Effect of Soil ph on Quackgrass Tissue Nutrient Content
Several important nutrient contents in quackgrass tissue changed with changes in soil pH, when averaged over all stages of growth until anthesis. Manganese concentrations were greater in both shoot (Table 11) and rhizome plus root tissue (Table 12) grown at eh lower soil pHs relative to that in the higher pHs. This same situation was true for boron in shoot tissue.Magnesium and phosphorus concentrations in both shoot and underground plant tissues were less in those grown at lower soil pHs compared to those at in higher pHs. The same situation was observed with copper and calcium in shoot, and zinc in rhizome plus root, tissues. Table 1. Effect of soil pH on quackgrass total shoot fresh weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP)
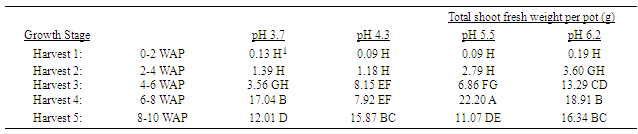 |
| |
|
Table 2. Effect of soil pH on quackgrass total shoot dry weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
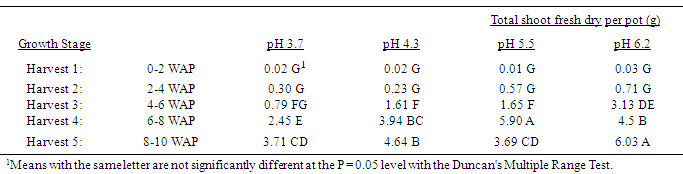 |
| |
|
Table 3. Effect of soil pH on quackgrass total rhizome plus root fresh weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
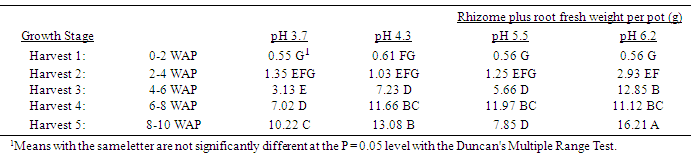 |
| |
|
Table 4. Effect of soil pH on quackgrass total rhizome plus root dry weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
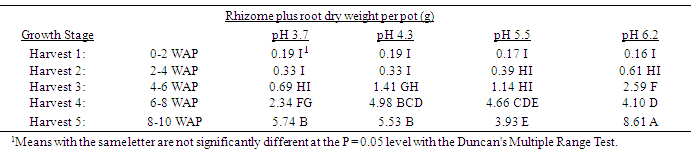 |
| |
|
Table 5. Effect of soil pH on quackgrass total shoot and rhizome plus root fresh weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 6. Effect of soil pH on quackgrass quackgrass total shoot and rhizome plus root dry weight per pot (g) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 7. Effect of soil pH on quackgrass total rhizome bud number per pot at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 8. Effect of soil pH on total quackgrass leaf numbers per pot at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 9. Effect of soil pH on total quackgrass shoot main axis numbers per pot at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 10. Effect of soil pH on quackgrass shoot height (tallest culm, leaf extended) (cm) at five harvests at several stages of growth; Harvest 1: 1-2 leaves per main axis (culm), 0 tillers with each main axis; Harvest 2: 2-4 leaves per main axis, 1-2 tillers with each main axis; Harvest 3: 2-4 leaves per main axis, 2-3 tillers with each main axis; Harvest 4: 2-4 leaves per main axis, 2-4 tillers with each main axis, with a minimum of 1 seedhead emerging per main axis; Harvest 5: 2-4 leaves per main axis, 3-5 tillers with each main axis, with a minimum of 1 seedhead at anthesis per main axis; weeks after planting (WAP).
 |
| |
|
Table 11. Effect of soil pH on quackgrass shoot nutrient concentrations (% of dry weight) averaged over five stages of growth: 1-4 leaves per main axis (culm); 0-5 tillers with each main axis, ending at anthesis; 0-10 weeks after planting.
 |
| |
|
Table 12. Effect of soil pH on quackgrass root plus rhizome nutrient concentrations (% of dry weight) averaged over five stages of growth: 1-4 leaves per main axis (culm); 0-5 tillers with each main axis, ending at anthesis; 0-10 weeks after planting; not statistically significant (N.S.).
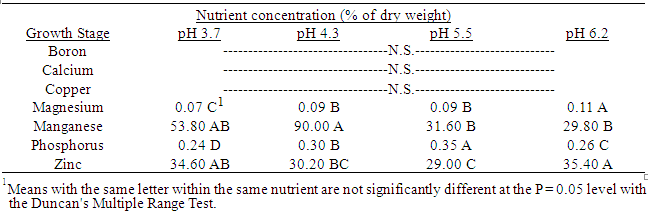 |
| |
|
4. Discussion
As the soil pH decreased in the range from 6.2 to 3.7, quackgrass plants accumulated less shoot, rhizome and root biomass, as well as less shoot height and numbers of main axis shoots, leaves and rhizome buds. This inhibitory effect of soil pH on quackgrass growth was most aparent in the later six weeks of development, until anthesis in the seedheads was apparent. In the first four weeks after planting the rhizomes, the reductions in quackgrass growth were best indicated by numbers of leaves and main axis shoots, as well as by shoot height. This reduction in growth associated with lower pH soils could be due to two mechanisms. The first mechanism could be interference with uptake and incorporation of magnesium and phosphorus into both above and below ground plant parts, as well as with copper and calcium in shoots, and zinc in rhizome and root tissue. A second mechanism could be due to toxicity caused by excessive amounts of manganese in all plant parts, as well as excessive boron in shoot plant parts. Both mechanisms probably operated to cause the observed growth reductions in quackgrass. The more acidic soils used in these experiments owed their low pH to the long term use of nitrogen fertilization in continuous corn cropping. Under these acid soil conditions, soil Ca, Mg, P and Zn became limiting to growth, while simultaneously Mn and B may have been present in excessive, toxic, amounts (Cook and Millar, 1953; Foth and Turk, 1972; Smith, 1952; Tisdale and Nelson, 1975).
References
| [1] | Cook, R. L. and C. E. Millar. 1953. Plant nutrient deficiencies. Michigan State College Ag. Exp. Sta. Special Bulletin 353, 1st revision |
| [2] | Dale, H. M., P. J. Harrison, and G. W. Thomson. 1965. Weeds as indicators of physical site characteristics in abandoned pastures. Can. J. Botany 43:1319-1327 |
| [3] | Dekker, J., H. MacKenzie and K. Chandler. 2006. The effects of soil pH on Setaria viridis and Abutilon theophrasti seedling growth and tissue nutrients. Agric. J. 1(1):1-4 |
| [4] | Foth, H. D. and L. M. Turk. 1972. Fundamentals of Soil Science. John Wiley and Sons, Toronto. 454 pp |
| [5] | LeFevre, P. 1956. Influence du milieu et des conditionss d'exploration sur le development des plantes adventices. Effet particutier du pH et l'etat clacique. Annales Agronomique (Paris) 7:299-347 |
| [6] | Smith, G. E. 1952. Soil fertility and corn production.. Missouri Agr. Ext. Sta. Bull. 583 |
| [7] | Thomas, R. L., R. W. Sheard, and J. R. Mayer. 1967. Comparison of conventional and automated procedures for nitrogen, phosphorus and potassium analysis of plant materials using a single digestion. Agron. J. 59:240-243 |
| [8] | Tisdale, S. L. and W. L. Nelson. 1975. Soil fertility and fertilizers, 3rd Edit. Macmillan Publ. Co., N.Y. 694 pp |
| [9] | Werner, P.A. and R. Rioux. 1977. The biology of Canadian weeds. 24. Agropyron repens (L.) Beauv. Can. J. Plant Sci. 57:905-919 |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML










