-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2011; 1(1): 1-12
doi:10.5923/j.re.20110101.01
Ecological Risk Assessment (ERA) as a Tool to Pollution Control of the Tanning Industry
Mwinyikione Mwinyihija
Kenya Leather Development Council, Nairobi, P O Box 14480 (00800), Kenya
Correspondence to: Mwinyikione Mwinyihija, Kenya Leather Development Council, Nairobi, P O Box 14480 (00800), Kenya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ecological Risk Assessment (ERA) is a tool vital to evaluate hazards and risks and eventually provide information that couldassist in averting and remedying the threats. ERA for the first time has been directed to the tanning industry focusing both at the processing line and occupational hazard to the workers. This review paper attempts to address these aspects by exploring novel techniques used recently through relevant research. For example Daphnia magna (primary consumer) and Escherichia coli HB101 pUCD607 (Primary decomposers) were used to evaluate the community structure. In addition supportive techniques were also used such as deterministic and probabilistic models. Results using ERA were very successful which yielded a tangible pathway to manage both the pollution generation during processing and waste management at the ‘end of the pipe’. It is also imperative to note that the study recognised the importance of integrating other techniques to further complement the reviewed approaches.
Keywords: Ecological Risk Assessment, Models, Primary Consumers, Secondary Consumers, Occupational Hazards, Waste Management
Cite this paper: Mwinyikione Mwinyihija, Ecological Risk Assessment (ERA) as a Tool to Pollution Control of the Tanning Industry, Resources and Environment, Vol. 1 No. 1, 2011, pp. 1-12. doi: 10.5923/j.re.20110101.01.
Article Outline
1. Introduction
- It is now common knowledge through studies particularly involving occupational health that indicate that direct contact with some industrial chemicals can cause disability, illness (toxigenic/carcinogenic) and death in humans [1]. In certain circumstances some minor exposures are known to cause the build-up sizeable toxic levels within living organisms. Some earlier studies by the reporting author have shown that solvents from degreasing and finishing are a source of exposure through vapors. Indeed simple aspects such day to day activities involving human health are vulnerable to toxic hazards. This has been observed by poor unskilled and unprotected handling of pesticides, tanning chemicals and treated hides, skins and leather. This was demonstrated through work done earlier where occupational hazards of the tanning industry were evaluated in details[2]. Other environmental hazards include visual impacts, excessive noise and air emissions closely associated with the leather sector.Evaluation of the ecological profile or better known as the Ecological risk assessment and for this purpose, of the tanning industry entails consideration of and understanding the categories of hazardous waste, its identification, exposure assessment, ecological effects and risk characterisation[3]. To carry out this exercise and be able to identify the toxicnature of the resultant tannery effluent and particulate matter from the atmosphere, specific bioassays (to evaluate responses to stressors) and chemical analysis (to provide information on the concentration and identification of the stressor) procedures were applied to ascertain the contributing factors.
1.1. Ecological Risk Assessment (ERA)
- Unless if anthropogenic in nature, chemical pollutants rarely attain acutely lethal concentrations in nature; thus the majority of their effects are expected to be sub-lethal. Estimation of the likelihood of effects from exposures to sub-lethal concentrations of contaminants in effluent plumes downstream of point sources poses a challenge when conducting ecological risk assessments (ERAs)[4]. In pursuance to this phenomenon the impact of the tanning industry will be evaluated through ecological risk assessment, which is a process that evaluates the probability that adverse ecological effects will occur as the result of exposure to one or more stressors. Studies conducted earlier have indicated that the objective of an ecological risk assessment is to determine and document actual or potential effects of contaminants. This approach is particularly directed to ecological receptors and habitats including fundamental basis for evaluating remedial alternatives. Ecological risk may be expressed as a probabilistic (Ecological Quantitative Risk Assessment (EQRA)) estimate of adverse effects (as in human health risk assessment), or may be expressed in a more qualitative manne[2,5,6].Quantitative risk assessment (QRA) has been a technique of choice used to estimate the probability of an adverse event[22]. The position of QRA is strengthened with an integration of Monte Carlo simulation. This approach has been found to offer precise explanation to the uncertainty and variability associated with the risk[23]. When this theorem was directed to the tanning industry it was purely to evolve the principles of ecological risk assessment. Thus to clearly articulate the definition of the ecosystem it can be portrayed as a grouping of organisms (microorganisms, plants, animal) interacting together, with and through their physical and chemical environment, to form a functional entity[24,25].Focusing on the true meaning therefore, the terminology related to risk assessment is not yet fixed. However after an initial statement of purpose[5,23,26], the process involves four primary stages described below in relation to the current paper:1. Hazard identification – literally in this phase it has been explained generally and by various authors also in different manner. However the most direct one cited by the current author[3] identifies the toxic chemical or substance of concern and moves a further stage to include whether the hazard actually is harmful. The problem-formulation process will involve the evaluation of the stressor (earlier defined by the author as a substance that has the inherent ability to impose adverse effects upon a biological system) characteristics, determines also if the ecosystem is at risk, and possible inherent ecological effects.2. Exposure assessment – this is stated as a second phase of approach to ERA and thus the same author in his earlier work, states that at this stage thrust is to determine the environmental concentration range of a particular stressor and estimating its rate of intake in target organisms. After establishing of the same the measurement will combine quantifiable indices that could be used describe the frequency and magnitude of contact. For future projections and development of tangible interventional goals mathematical models will be formulated to ascertain the resultant exposure to a stressor and to determine the outcome of a variety of scenarios. 3. Ecological effect is the next crucial stage which has to integrate toxicity assessments. To elucidate the factors affecting this particular aspects warrant the need to identify and quantify the adverse effects elicited by a stressor. Subsequently this will than make it easy to determine the cause-and-effect relationship detected in various ecologically threatened conditions. Resultant data include acute toxicity data which forms the basis of primary input needed during the simulation stage of the developed model.4. Risk characterization – as a final input to the model this phase includes comparison of the exposure and stressor- response profiles to determine probabilistic effects taking into cognizance the contaminant and the predisposed population in the set scenario.
1.2. Bioassays
- Bioassays involve usage of biotics (living organism) in controlled environment, responses towards xenobiotics, pollutants or contaminants in the environment (e.g. Phytotoxicity, bioluminescence response etc.)[3,27]. Bioassays have larger variability than most chemical analysis due to biological variation. The major advantage of bioassays is that the total toxicity of wastewater can be assessed by taking into account bioavailability and synergistic or antagonistic effects. Also, transforming information on concentration to information on biological response is useful for risk assessment. Chemical analysis provides information on the concentration of a substance in a sample and may help to identify that substance[28]. However, this does not give direct information relating to the bioavailability and impact of environmental pollutants.
1.3. Biomass Activity
- Monitoring and evaluation of biomass activity (e.g. intracellular enzyme activity) has been established[17] at the river sediment level, the basis of this study was the provision of vital information on stressor-response and ecological effects. More advanced has well been articulated and demonstrated that knowledge related to the reduction of sediment capacity to act as a fully functioning mineralization medium for natural pollutant substrates (Figure 1) is critical in overall river health assessment[29].
 | Figure 1. Correlation to Mineralisation of natural pollutant substrate and biosensor response[29] |
1.4. Weight of Evidence in Ecological Risk Assessment
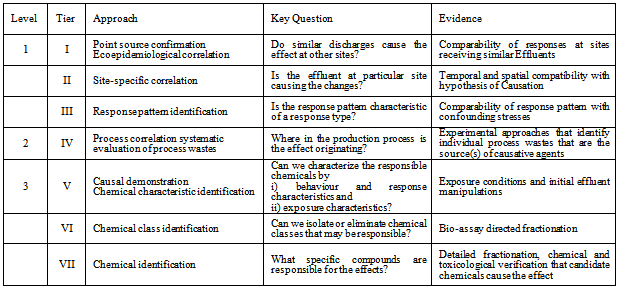
- When carrying out weight of evidence in any ERA programmes it becomes quite necessary to utilise well defined pathways for an all inclusive risk assessment. One of such technique is the use of multiple criteria or multiple lines of evidence (also referred to as weight of evidence). This is reported in this paper and essentially used earlier in determining the ecological systems[7,8] and even recommended in evaluating risk to the environment[9-13]. Drawing from earlier presentation the following criterions such as Strength of association; Consistency of association; Specificity of association; Time order or temporality; Biological gradient; Experimental evidence and Biological plausibility, were chosen for application during preparation of this paper. This is because they have already been established in this area[14], and are readily applicable to environmental samples sourced from the Kenyan tanning industry. A linkage was developed using the three levels and seven tiers reported[15] (Table 1), involved in a casual identification, including identification of effects, development of correlative relationships and confirmation that specific chemicals are responsible for the observed effects.
1.5. ERA–Query Points
- Two questions were developed to develop the Ecological Risk Assessment (ERA): (1) has the water quality of the river been affected by the discharged tannery effluent? and (2) has the river sediment health been affected by the deposition of the contaminants?
2. Discussion
2.1. Approach to Ecological Risk Assessment (ERA)
- This paper envisages to discuss the ecological risk caused by the leather sector particularly its tannery industry. It is envisaged that this will be achieved by responding to the risk questions or query points raised here-above. In lieu therefore there is a dire need to establish attainable but well defined criterions as follows:i) Identify stressors/hazards.ii) Apply biological technique (e.g. Bioassay) and chemical assays (to determine heavy metals, COD, BOD, Total Phenols etc) – this for the purpose of determining the stressors and resultant effects to the environment. iii) Determine through probabilistic models that the stressors (toxic chemicals from the tanning industry) cause quantified impact to the ecosystems.iv) Ascertain the resultant impact of the risk mitigation put in place to curtail the ecological contamination risk.Ecological evaluations require optimal techniques to determine potential impacts and risks. Therefore this study focused on risk assessments applying bioassays as a novel technique were a series of different scenarios integrating time as a factor to exposure of the bio-entity in identified system to the hazard, toxicity levels were conducted at known sampling points and bioluminescence evaluation determined on application of a genetically modified biosensor. The mentioned parameters was integrated and run using the deterministic model. Inclusively, chemical, identified modulation programme and protocols defined by the environmental agency in the US including capturing aspects related to acute toxicity in effluents[9-12] were adopted to ascertain ecological risk. Collection of experimental samples was obtained from both river sources and tannery lagoons. This approach of ascertaining ERA’s also include the study of multiple stressors to provide an in-depth evaluation[3,16].The investigation entailed the effluent treatment phase which started from raw effluent through treated effluent like general sedimentation and final effluent where for this case was carried out using anaerobic lagoons, explored upstream and downstream of the riverine by carrying out sampling which eventually provided the basis of risk assessment. To strengthen the statistical position of the study it was important to ensure that it was random and repeated eight times over a period of sixteen weeks. Parameters recorded included observations on visual (water colour, vertebrates, plants etc) and observable odorous peculiarities. Moreover to strengthen our scientific investigation biological and chemical investigations were performed to identify the class or causative aspects of the pollutants or contaminants involved.
|
2.2. Approach to Data Analysis
- To articulate the various scenarios carried out, the risk assessment model was run applying the deterministic model as indicated in Table 2.
|
2.3. Preview of Risk Assessment and Identification
2.3.1. Hazard Identification and Problem Formulation
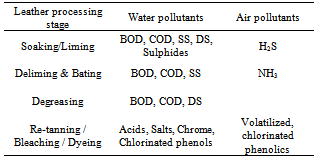
- Within the leather sector it was very important in hazard identification and problem formulation certain crucial aspects need to be considered. Table 4 depicts the processing stage, water pollutants and air pollutants and average trend in effluent produced over time as indicated in Figure 2.i.) 1st stage of leather processing - soaking activitiesAn important stage and the most preliminary of the processes in the tanning industry is the soaking process. The processing of skins and hides at this stage targets cleaning, rehydration of the material, conditioning and facilitate attainment of appropriate moisture content. Once achieved than subsequent processes related to liming, deliming and pickling as important stages of beam house operations. Because of many of the raw material being wet salted apparently results in production of salts. Other debri or contaminants are also associated with free ammonia, mal-odours, soil related dirt, blood, dung and other related physico materials related to the bio chemicals and synthetics (e.g. wetting agents, surfactants, emulsifiers etc) which, are released during the tanning process.
|
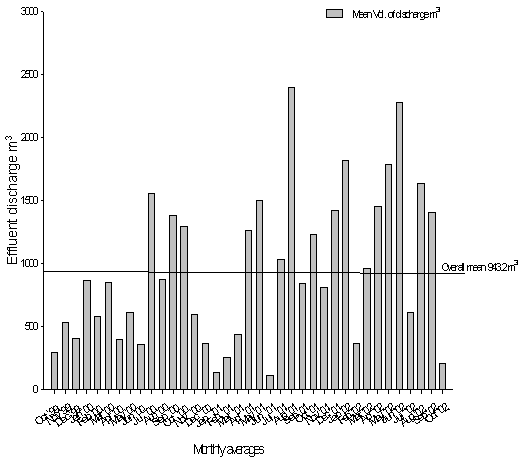 | Figure 2. Tannery effluent mean monthly discharge 1999 – 2002 at a Kenyan site adopted[3] |
|
|
2.2. Description of Exposure Assessment
2.2.1. First Line of Action-End-Points and Conceptual Model
- To achieve end points and develop a conceptual model it is important to screen risk assessment. It is also important to include and review ecotoxicity literature review, and the complete exposure pathways, development of a conceptual model. In deed this is a an approach also used[35] when conducting exposure studies for threatened bull trout and rainbow trout. However, for the leather industry two main contaminants of the effluent (Chromium and Phenols) were earmarked, known to influence acutely or chronically toxically organism within an aquatic community. This was in the basis that direct exposure of the untreated effluent to the river was found to cause acute or chronic toxicity to the overall aquatic zoo and phyto organisms. The current status of the effluent after treatment was determined and evaluated for contents toxicity. The exposure pathways were therefore evaluated through direct contact with contaminated sediments and water. Daphnia magna (primary consumer) and Escherichia coli HB101 pUCD607 (Primary decomposers) were used to evaluate the community structure.As was indicated in earlier paragraphs it is imperative to complement the bioassay test with chemical evaluation technique. As such river sediments were also analysed for heavy metals (Table 6). To determine the diffusion potential the speed and the depth of the river at the discharge point (1.17 m s-1 (high water levels –rainy seasons) to 0.513 m s-1 (reduced water flow period low rainy seasons)) were observed[3]. This inclusion brought to the fore the importance of river dynamics and dilution factor of the river.
|
|
2.3. Outline of Ecological Risk Characterization
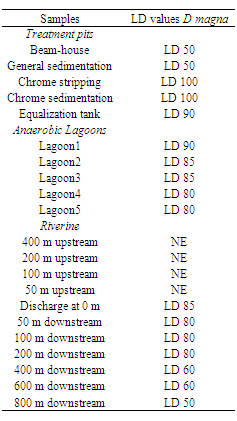
- This phase comprised the comparison between exposure and stressor-response profiles to estimate the probability of effects, using the distribution or magnitude of the stressor in the areas sampled. When reviewing this work a link identified the ‘cause and effects’ (as shown in Table 1). This is further elaborated and established using correlative relationship and specific chemicals to determine the observed stressor effects[15] (Table 1).Thus author in reference made an interesting observation where he conclude that ‘the decrease in percentage bioluminescence (64%), dehydrogenise activity (0.0058 μgTFg-1 sediment 6 h-1) and daphnia count (LD 85) values at the discharge point, and immediately thereafter downstream indicated the impact of the stressor to the riverine ecosystems’. However, to comment on the same further it was observed that no effect was observed upstream, demonstrating the possible source of contamination emanated from the tannery discharge point. In retrospect, similarly, chemical analysis showed that an increase of the stressor at the discharge point when compared to areas upstream and extreme sampling points downstream (Table 6 and 7).
2.4. Potential Ecological Risk Mitigation Suggested
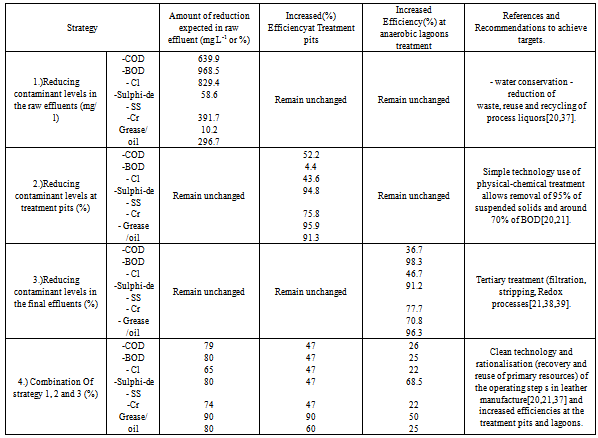
- Leather processing generally involves a combination of single and multi-step processes that employ as well as expel several biological, organic and inorganic materials[36]. As a follow-up in the review of this paper, base values (Table 2) were determined and model variables identified (Table 3) after the tannery effluents were analysed (Table 5). This was necessitated by the requirement to reveal in-plant methods of reducing the quantity and quality of the wastewater. By the beginning of the investigation, it was anticipated that there would be a reduction of the pollution load from the tannery. The result associated would therefore mean that there will be an improvement on water, energy and chemical consumption[21]. Hence, appropriate mitigation strategies are strongly envisaged for purposes of addressing complexes which were performed through an importance analysis using a deterministic model. The different scenarios (Table 2) are now included as probable mitigation strategies (Table 9). The reduction of contaminants and or increase in treatment efficiencies was also manipulated to meet target requirements in the effluent composition (Table 8).
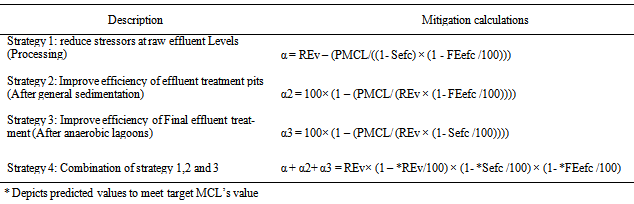
2.4.1. Description of Mitigation Strategies
- Certain mitigation has been developed to fit into the ERA redirected towards the leather industry. To achieve this a deterministic model was found to be able to amend so as to take into account a single input parameter change or alternately a change in one of the assumptions upon which the model is based. Essentially this was important when appraising the hypothetical risk mitigation strategies indicated in Table 9. These strategies can be implemented in the model and the change in the output (i.e. to meet set out domestic use in related maximum contaminant levels guideline for identified tannery effluent pollutants discussed in part I of an earlier paper) can be calculated to determine whether it is feasible or not. The following strategies were considered as detailed in a study by[17];
|
2.4.2. Adopted Mitigation Calculation
- The applicability and effectiveness of risk mitigation strategies (Table 9) was attained after performing a deterministic model for the scenarios detailed in Table 2 and variables in Table 3. The effectiveness and formulation of each individual mitigation strategy used a calculation approach shown as per the description indicated in Table 10.
|
|
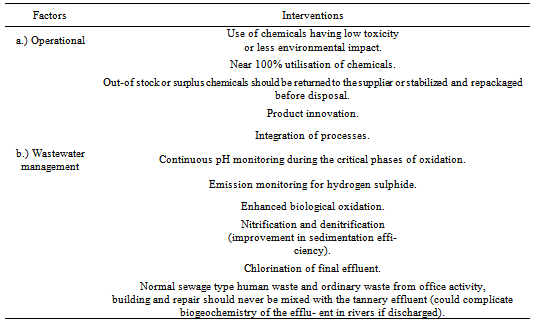
2.4.3. Achieving the Targets towards Mitigation Strategies
- The author found out that in achieving the set mitigation strategies there were two principal approaches i.e. operational and waste management factors (Table 11) that formed a fundamental basis if developing ERA. To fathom the idea further the study came up with three main domains which literally incorporated rationalisation through risk assessment model development; leather processing (raw effluent); increasing efficiencies during effluent treatment e.g. chrome stripping, effluent equalisation/sedimentation and final effluent treatment directed towards aerobic or anaerobic lagoons as detailed earlier on in this paper.
|
3. Conclusions
- This paper attempted to preview the essentials of ERA as a viable tool, taking a holistic approach on earlier versions that were used to evaluate risks e.g. QSAR, QRA etc. For instance, it would be noted that either the techniques mentioned here-above purely used mathematical approaches or used only chemical analysis. However in this study a new approach which targeted the tanning industry has been formulated. In assessing the impacts all the crucial stages of the tanning industry were involved. This approach assisted in identifying all the stressors in the value chain. Essentially therefore individual levels were ascertained giving the model developed an independent interventional strategy. Most intriguing though was the tremendous achievement attained by the author when he developed different strategies from various processing points. Although the effectiveness and efficiencies were varied at each stage, the use of all the strategies using the mathematical formulation indicated under Table 10 was opportune. In particular there was a reduction of the stressor level at the end of the treatment phase. Categorisation and partitioning of the contaminant load during the processing of leather was important and very fundamental. For example the study clearly indicated that the most prone area that caused high levels of pollution was the beam house than any other place in the leather processing phases. The demonstration of the models has heralded a new frontier in the leather sector where not only can we predict the levels and points prone to contaminant load but also assist in developing strategies to remediate and manage the environmental concern of the tanning process worldwide. The critical area that may need to be integrated is the LCA (Life Cycle Assessment) so as to make the technique of ERA not only in managing the leather processing but also accountable to the environmental impact accruable from leather products developed and its associated lifespan.
ACKNOWLEDGEMENTS
- To all staff of Atlantic International University (AIU) for their support during the post-doctorate studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML