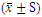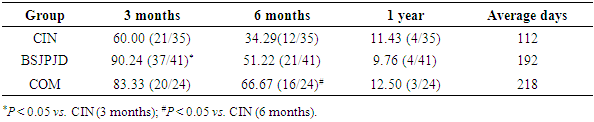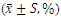-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2025; 12(1): 7-13
doi:10.5923/j.rct.20251201.02
Received: May 26, 2025; Accepted: Jun. 22, 2025; Published: Jul. 23, 2025

Clinical Effect Observation of Bushen-Jianpi-Jiedu Formula and Its Combination with Cinobufacini on Postoperative Colorectal Cancer
Xueqing Hu 1, 2, Mengdie Yang 2, Xiaole Chen 2, Wenjun Zhou 2, Qing Ji 1, 2, Yang Wang 1, 3, Shibing Su 2
1Department of Medical Oncology, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
2Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
3The Second Clinical Medical College&, The Second Affiliated Hospital, Guizhou University of Traditional Chinese Medicine, Guiyang, 550002, China
Correspondence to: Yang Wang, Department of Medical Oncology, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China; Shibing Su, Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China.
| Email: |  |
Copyright © 2025 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: To observe the clinical efficacy of Bushen-Jianpi-Jiedu formula (BSJPJD), Cinobufacini injection (CIN) and their combination (COM) on the postoperative colorectal cancer (CRC) patients. Methods: A total of 100 postoperative CRC cases were enrolled and randomized to the groups of CIN (35 cases), BSJPJD (41 cases) and COM (24 cases). The clinical effects on progression-free survival (PFS), Traditional Chinese Medicine (TCM) syndrome, quality of life, immune function as well as side effects were evaluated in each group. Results: the BSJPJD group and COM group had the highest PFS rates at the 3 and 6 months, respectively (P < 0.05). The improvement rate on TCM syndrome was higher in BSJPJD group and COM group than that in CIN group (P < 0.05). CIN treatment increased the CD4+ T cell proportion (P < 0.05). COM group has higher diarrhea incidence than CIN group (P < 0.05). Conclusion: BSJPJD prolonged the PFS of CRC patients at 3 months. CIN could better modulate the immunity. COM group prolonged the survival of CRC patients at 6 months, but may cause diarrhea.
Keywords: Bushen-Jianpi-Jiedu Formula, Cinobufacini, Clinical observation, Colorectal cancer
Cite this paper: Xueqing Hu , Mengdie Yang , Xiaole Chen , Wenjun Zhou , Qing Ji , Yang Wang , Shibing Su , Clinical Effect Observation of Bushen-Jianpi-Jiedu Formula and Its Combination with Cinobufacini on Postoperative Colorectal Cancer, Research In Cancer and Tumor, Vol. 12 No. 1, 2025, pp. 7-13. doi: 10.5923/j.rct.20251201.02.
Article Outline
1. Introduction
- Colorectal cancer (CRC) is one of the common malignant tumors in clinical practice, and new cases of CRC are rising annually [1,2]. Clinically, surgery is the treatment of choice for CRC, but about 50% of CRC patients develop metastasis within 2 years after radical surgery. The 5-year survival rate of patients with non-metastatic CRC is higher than 75%, while the 5-year survival rate of patients with metastatic CRC is only 10%, and clinical studies have shown that more than 90% of CRC patients die from metastasis [3-5]. Therefore, the prevention of CRC metastasis is of great significance to improve the survival rate of CRC patients in China.In recent years, research into anti-tumor drugs has made notable progress, encompassing the design, synthesis, characterization, and biological activity assessment of various compounds. It also includes exploring new synthetic methods (such as microwave-assisted synthesis) and discovering compounds with unique structures (like fan-shaped and sperm-like amphiphilic heterocyclic compounds). This underscores the pursuit of structural diversity and functional specificity in modern chemical synthesis and drug development [6-13]. However, these studies fall short in evaluating compound safety-critical indicators like toxicity and side effects are not adequately addressed, impeding the translation of drugs from lab to clinic. Furthermore, the lack of in vivo validation makes it hard to fully capture the real efficacy of these compounds in living organisms and leaves them ill-equipped to tackle the highly complex mechanisms underlying tumorigenesis and progression.Traditional Chinese medicine (TCM) -based adjuvant therapy can improve complications such as intestinal obstruction in postoperative CRC patients, improve patients' immunity, and prevent their recurrence and metastasis [14]. Systematic evaluation and Meta-analysis showed that Jianpi formula can improve the survival quality of postoperative CRC patients, reduce the toxic side effects such as leukopenia, platelet lowering, nausea and vomiting induced by chemotherapeutic drugs [15]. Modified Si-Jun-Zi Decoction can improve the clinical efficacy of chemotherapy in postoperative CRC patients, reduce their adverse reactions, and improve their quality of life [16]. Modified Xiangsha-Liujunzi Decoction can reduce the recurrence rate and metastasis rate of postoperative CRC patients and prolong the survival period of patients [17]. Modified Shen-Ling-Baizhu San combined with mannan peptide significantly improved the immune function and clinical symptoms of patients after postoperative chemotherapy for CRC [18]. BSJPJD is a clinically formula in the Department of Oncology, Shuguang Hospital of the Shanghai University of Traditional Chinese Medicine. It is suitable for the prevention and treatment of recurrence and metastasis in postoperative cancers including CRC under the guidance of TCM theory. Cinobufacini injection (CIN) is more effective against advanced malignant tumors of the digestive system [19]. Therefore, this study observed the effects of BSJPJD, CIN and its combination on disease progression-free survival (PFS) and quality of life, immune function and adverse reactions in postoperative CRC patients.
2. Materials and Methods
2.1. Clinical Information of Enrolled CRC Patients
- The study was conducted from March 2014 to February 2018, and a total of 100 postoperative CRC patients were enrolled from Shuguang Hospital, Shanghai University of Traditional Chinese Medicine. All experimental protocols complied with the Guidelines for Ethical Review of Drug Clinical Trials, the Declaration of Helsinki, and the International Ethical Guidelines for Biomedical Research Involving Human Beings (CIOMS: 2002). The project was reviewed and approved by the Ethics Committee of Shuguang Hospital (NO. 2014-345-41-01). Postoperative CRC patients were divided into the CIN treatment group, the BSJPJD group, and the combination treatment (COM) group randomly.
2.2. Diagnostic Criteria
- CRC diagnosis was confirmed by postoperative pathology according to the Guidelines for the diagnosis and comprehensive treatment of CRC liver metastases (2016) [20].
2.3. Inclusion Criteria
- According to the clinical TCM syndrome and medication, postoperative CRC patients were included in this study, and the specific inclusion criteria were as follows: (1) those who had no metastatic lesions after CRC; (2) those who were identified as liver and kidney Yin deficiency, spleen and stomach Qi deficiency in TCM. The diagnostic criteria were referred to Guidelines for Clinical Research of New Traditional Chinese Medicines (Third Series). Liver-kidney Yin deficiency, spleen-stomach Qi deficiency were manifested by rib pain, lumbar and knee soreness, hot flashes and night sweats, dry mouth and throat, fatigue, nausea and more serious abdominal distension after eating, with a pale or red tongue, scanty or light moss, and a sunken and numb pulse; (3) aged 18-80 years old, male or female, with Karnofsky Score (KPS) ≥ 60 [21], and with an expected survival period of ≥ 3 months; and (4) signed an informed consent form.
2.4. Exclusion Criteria
- Exclusion criteria: (1) patients with serious cardiac, renal, hematopoietic system disorders and other factors affecting drug evaluation; (2) patients with mental disorders; (3) patients with digestive tract obstruction; (4) pregnant and lactating women; (5) people who use drugs other than those specified in the program; (6) people who are allergic to the study drugs or ingredients; (7) people with poor compliance.
2.5. Treatment
- CIN treatment: 20 mL of CIN were diluted into 500 mL of 5% glucose and were given intravenously once a day. The course of treatment was two weeks, after four weeks the patient rested for one day. The total duration of treatment is 3 months. BSJPJD group: BSJDJD formula consists of Di Huang (Radix Rehmanniae), Ba Yue Zha (Rhizoma Augustiniae), Ye Pu Tao Teng (Radix et Rhizoma Dioscoreae) etc. and was supervised by Shuguang Hospital of Shanghai University of Traditional Chinese Medicine and produced by Jiangyin Tianjiang Pharmaceuticals Co Ltd (batch no.: 1611328). CRC patients took two sachets twice a day. Each sachet contained 8.95 g of herbs and was given for 30 days continuously. This study was conducted for 3 consecutive courses. The drugs were distributed by the Pharmacy Department of Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, and quality control was performed. COM group: Dosage and course of treatment were same as above.
2.6. Observation Indicators and Efficacy Evaluation
- Survival observationAfter the end of treatment, patients were followed up monthly until progression or death, with a follow-up date of June 31, 2018 for all patients included in the survival assessment. Patients who did not die were used as the cut-off value, which was the time of the last follow-up visit to confirm non-death. No patients were lost to follow-up. Disease progression-free survival at 3 months, 6 months, and 1 year was analyzed for postoperative CRC patients based on survival data.Evaluation of the efficacy on TCM symptomsReferring to the “Guidelines for Clinical Research of New Chinese Medicines” formulated by the Ministry of Health in 2002, combined with clinical practice, the criteria for TCM evidence points and TCM evidence efficacy were formulated as follows: TCM evidence points were set at 0, 1, 2, 3 according to none, light, moderate, and heavy respectively. The efficacy of TCM points: significant improvement: decrease in points ≥ 2/3; partial improvement: 1/3 ≤ decrease in points < 2/3; no improvement: decrease in points < 1/3.Quality of life assessmentKPS Functional Scale: 100 points: normal, no symptoms, no abnormal signs; 90 points: normal activity with mild symptoms and abnormal signs; 80 points: normal activity with some symptoms and abnormal signs; 70 points: self-care, but unable to maintain normal life and work; 60 points: mostly self-care with occasional need for help; 50 points: needs constant care; 40 points: unable to care for himself/herself, needs special care and help; 30 points: severely unable to care for himself/herself; 20 points: severely ill, needs hospitalization and active supportive treatment; 10 points: seriously ill, near death; 0 points: death. The lower the score, the worse the health condition [22].Immune cell testingFlow cytometry was used to detect the number of peripheral blood T-cell subpopulations CD3+, CD4+ and CD8+ as well as the number of NK cells, which were tested by the laboratory department of Shuguang Hospital.Evaluation of adverse reactionsHematologic and non-hematologic adverse reactions were evaluated with reference to the “Criteria for the Presentation and Classification of Acute and Subacute Toxic Side Effects” published by the World Health Organization in 1970.
2.7. Statistical Methods
- SPSS 21.0 software was used for statistics. If the measured data conformed to normal distribution, the t-test was used to compare two groups. The one-way ANOVA test was used to compare three groups, and the non-parametric test was used if the data did not conform to normal distribution. The chi-square test was used for count data, and the Kaplan-Meier method was used for survival curves.
3. Results
3.1. Clinical Information of Postoperative CRC Patients
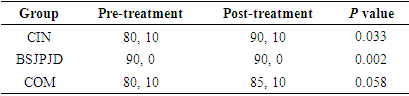
- A total of 100 postoperative CRC patients were enrolled and randomly divided into 35 cases in the CIN group, 41 cases in BSJPJD group, and 24 cases in the COM group. There was no statistically significant difference in age, gender, pathological stage and immune status of patients in each group (P > 0.05) (Table 1).
|
3.2. Effect on PFS of Postoperative CRC Patients
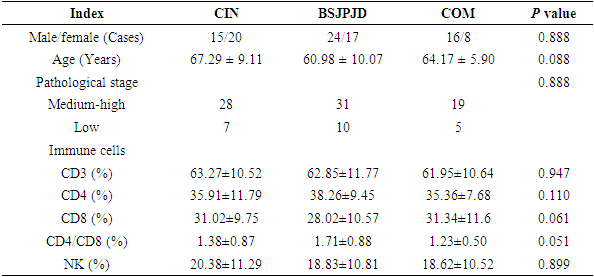
- The differences in the effects of the three treatment regimens on 3-month and 6-month’s PFS of CRC patients were statistically significant (P < 0.05). The 3-month’s PFS of the BSJPJD group was higher than that of the CIN treatment group (P < 0.05), and the difference was not statistically significant compared with that of the COM treatment group (P > 0.05). The 6-month’s PFS of the COM treatment group was higher than that of the CIN group (P < 0.05), and the difference was not statistically significant compared with that of the BSJPJD group (P > 0.05), and the difference in the 1-year disease FPS of the three treatment regimens was not statistically significant (P > 0.05) (Table 2). The overall PFS of the COM group was higher than that of the BSJPJD treatment group, and overall PFS of the BSJPJD group was higher than that of the CIN group (Figure 1).
|
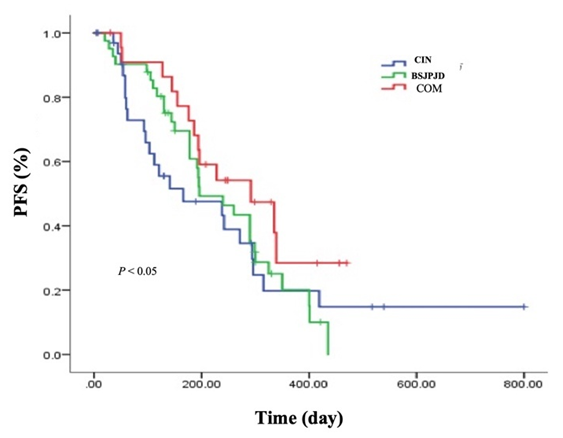 | Figure 1. PFS of patients after different treatment regimens |
3.3. Improvement of the TCM Symptoms in Postoperative CRC Patients
- The differences in the overall distribution of the degree of improvement in TCM symptoms and the total improvement rate of the three treatment regimens were statistically significant (P < 0.05). The rate of significant improvement of TCM symptoms in the COM group was higher than that in the CIN group and the BSJPJD group (P < 0.05). The rate of partial improvement in the BSJPJD group was higher than that in the CIN group (P < 0.05). The improvement rate of TCM syndrome points in the COM group was higher than that in the CIN group (P < 0.05), and there was no statistically significant difference between the CIN group and the BSJPJD treatment group (P > 0.05).
|
3.4. Effects on the Quality of life of CRC Patients
- Both the CIN group and the BSJPJD treatment increased patients’ KPS scores (P < 0.05). The difference in KPS score increase between the BSJPJD group and the CIN treatment group was statistically significant, indicating that the BSJPJD improved the quality of life better than CIN (P < 0.05).
|
3.5. Effect on Immune Cell Composition of CRC Patients
- There was no statistically significant difference in the proportion of CD3+ cells, CD8+ cells, NK cells and CD4+/CD8+ ratio before and after treatment with CIN, BSJPJD and COM groups (P > 0.05). The CD4+ cell ratio was increased after treatment with CIN (P < 0.05), while the difference in CD4+ cell ratio was not statistically significant before and after treatment with the BSJPJD and the COM groups (P > 0.05).
|
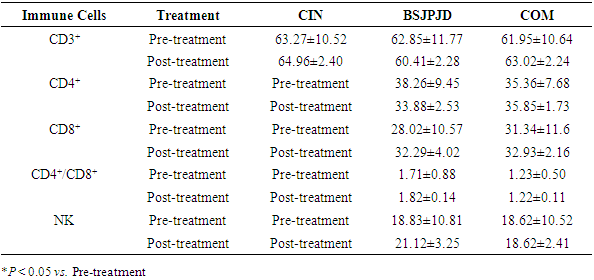
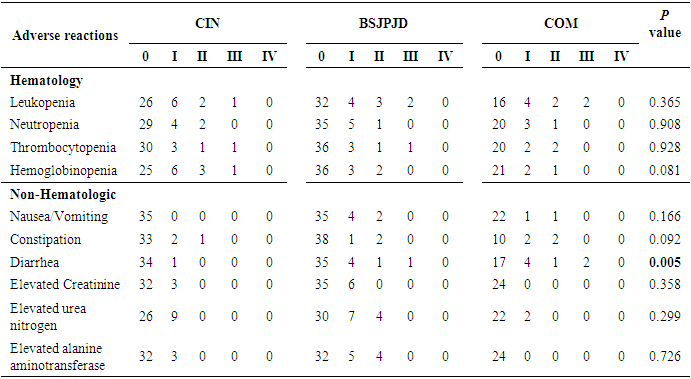
3.6. Adverse Reactions
- The difference in the overall distribution of diarrhea among the three treatment regimens was statistically significant (P < 0.05), and the incidence of diarrhea in the COM treatment group was higher than that in the CIN group (29.17% vs. 2.86%, P < 0.05), suggesting that the COM group was more likely to cause diarrhea than CIN alone. The difference in the incidence of diarrhea between the CIN group and the BSJPJD group was not statistically significant (2.86% vs. 14.63%, P > 0.05). The overall distribution of other non-hematological and hematological adverse reactions was not statistically different among the three treatment regimens (P > 0.05) (Table 6).
|
4. Discussion
- As one of the most common malignant tumors worldwide, CRC remains a significant challenge in terms of treatment. Its high recurrence rate and marked differences in sensitivity to chemotherapy and surgery make individualized treatment difficult to implement. Additionally, the five-year survival rate for CRC is still relatively low (32%), reflecting the limitations of current treatment methods [3-5]. Research indicates that the mechanisms behind CRC development are not yet fully elucidated, which increases the difficulty of targeted therapy and leaves effective drug targets still limited.TCM has shown significant advantages in the treatment of CRC. Extensive clinical studies have demonstrated that the integration of TCM with conventional medicine can significantly improve patient survival quality and alleviate symptoms, particularly among advanced patients [23]. Furthermore, TCM has unique advantages in enhancing patient mental health and overall quality of life, which is particularly crucial for patients with recurrent CRC [24].The role of TCM in the prevention and treatment of CRC has been widely reported. Bushen-Jianpi formula can improve the symptoms and quality of life of patients with liver and kidney Yin deficiency and spleen Qi deficiency after hepatocellular carcinoma surgery [25], and also inhibit the growth of subcutaneous graft tumors in nude mice with human colon cancer and prolong the survival time of rats bearing tumors [26]. A multicenter randomized controlled clinical study found that a Jianpi-Jiedu receipe significantly improved quality of life and prolonged median survival in patients with advanced CRC [27,28].Our clinical observation on survival, quality of life, immune function and adverse reactions of postoperative CRC patients revealed BSJPJD can improve the PFS of CRC patients for 3 months, BSJPJD combined with CIN can improve the PFS for 6 months. However, there was no statistically significant difference in the 1-year survival rates among the three treatment regimens, suggesting that BSJPJD could achieve short-term clinical efficacy. The combination regimen was more effective than the BSJPJD group or CIN group, but it was prone to cause adverse reactions such as diarrhea. Therefore, the patient's status and tolerance to adverse effects should be considered when combining the drugs. BSJPJD formula enhanced the effect of improving the quality of life better than that of CIN, which can increase CD4+ immune cells, and CD4+ T cells can initiate cytotoxic T lymphocytes through multiple pathways to exert anti-tumor effects. Therefore, the regulation of cellular immunity in postoperative CRC patients by CIN is better than that by BSJPJD formula. Therefore, BSJPJD formula and CIN exert clinical efficacy from different aspects.In this study, we initially observed the effects of BSJPJD, CIN and its combination on survival and quality of life, immune function and adverse reactions of postoperative CRC patients. Due to the small number of enrolled cases and the occurrence of adverse reactions such as diarrhea in patients, there was a loss of cases during this study, and the results of this study need to be further verified by larger sample size. Further studies are needed to investigate the mechanism of improving PFS of postoperative CRC patients by BSJPJD, as well as the mechanism of adverse effects of combining this formula with CIN. In addition, since the efficacy of TCM is highly dependent on individual differences, it remains an important challenge to establish uniform assessment standards and operational protocols.
ACKNOWLEDGEMENTS
- The sutpdy was suppoted by the National Natural Science Foundation of China (81330084) and Shanghai Pujiang Program (23PJ1412400).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML