-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2025; 12(1): 1-6
doi:10.5923/j.rct.20251201.01
Received: Dec. 29, 2024; Accepted: Jan. 15, 2025; Published: Jan. 18, 2025

Endometrial Hyperplasia in the Preoperative Preparation of Menopausal Women: Ultrasound Visualization Techniques
Ya. M. Mamadalieva, L. E. Shamsieva
Center for the Development of Professional Qualifications of Medical Workers under the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to determine the sequence of multiparametric examination of endometrial cancer depending on tumor size, degree of invasion, clinical picture with subsequent histological verification at the stage of preoperative preparation. Background. Endometrial malignancy is one of the most frequent tumors of the female reproductive organs. Statistics show that endometrial cancer is developed in 200,000 women each year, with a mortality rate of 50,000 women per year. The incidence of uterine cancer in developed countries is 12.9/100,000 (mortality rates 1.6/100,000), whereas in undeveloped countries it is 5.7/100,000 (mortality rates 0.7/100,000). Material and methods. The study included 68 women with complaints of postmenopausal bleeding and dysfunctional vaginal bleeding in the anamnesis during hormone replacement therapy. The age of patients ranged from 48 to 77 years. All women were examined according to the general scheme: anamnestic data collection, gynecologic examination, risk factor assessment, comprehensive ultrasound examination of pelvic organs, internal organs and retroperitoneal lymph nodes. Results. We analyzed the results of multiparametric ultrasound in seroscale mode, color Doppler mapping mode and elastography mode: compression elastography and shear wave elastography, performed in patients with endometrial hyperplastic process at the stage of preoperative preparation. The list of concomitant diseases of the patients was presented. The changes in the echographic picture and hemodynamic parameters of the altered endometrium depending on the histotype of pathology and the presence of myometrial invasion were revealed. Conclusion. This study revealed that the complex application of multiparametric ultrasound techniques (B-mode combined with color Doppler mapping) was highly informative and should be used in the differential diagnostics of endometrial hyperplastic processes in the preoperative period in pre- and postmenopausal women with a history of dysfunctional vaginal bleeding.
Keywords: Endometrial cancer, Ultrasound diagnosis, Myometrial invasion, Endometrial Doppler, Neoangiogenesis
Cite this paper: Ya. M. Mamadalieva, L. E. Shamsieva, Endometrial Hyperplasia in the Preoperative Preparation of Menopausal Women: Ultrasound Visualization Techniques, Research In Cancer and Tumor, Vol. 12 No. 1, 2025, pp. 1-6. doi: 10.5923/j.rct.20251201.01.
Article Outline
1. Introduction
- Endometrial malignancy is one of the most frequent tumors of the female reproductive organs. Statistics show that endometrial cancer is developed in 200,000 women each year, with a mortality rate of 50,000 women per year [1]. The incidence of uterine cancer in developed countries is 12.9/ 100,000 (mortality rates 1.6/100,000), whereas in undeveloped countries it is 5.7/100,000 (mortality rates 0.7/100,000) [2].The incidence of endometrial carcinoma increases with age (5-10 years before menopause) with the incidence peaking between 65 and 70 years [3].The majority of patients diagnosed with endometrial cancer (EC) are postmenopausal women, with an average age of 68 years at the time of diagnosis. However, up to 15% of cases may occur in premenopausal women [4].Endometrial carcinoma is usually manifested by abnormal uterine bleeding in postmenopausal women, and the diagnosis is confirmed by evaluation of an endometrial scraping biopsy or hysterectomy specimen [5].Traditionally, endometrial cancer is classified into two pathogenetic variants based on clinical and hormonal features with different pathophysiologic and histologic features.Prognosis depends on the age of the patient, histological type of malignancy, tumor size, metastasis to regional and retroperitoneal lymph nodes, depth of invasion into the myometrium and involvement of the cervix in the process [6].Tumor size, depth of myometrial invasion and cervical stromal involvement cannot be determined on clinical examination. Thus, multiparametric ultrasound and magnetic resonance imaging (MRI) are increasingly being used to improve preoperative evaluation, i.e., to identify women requiring more extensive surgical intervention, including pelvic and para-aortic lymph node dissection. According to the literature, systematic use of echography contributes to earlier diagnostics of endometrial cancer, increasing the incidence of stage I cancer by 50% and reducing the incidence of stage III-IV cancer by 1.5 times [7,8].The aim of the study was to determine the sequence of multiparametric examination of endometrial cancer depending on tumor size, degree of invasion, clinical picture with subsequent histological verification at the stage of preoperative preparation.
2. Material and Methods
- Women treated in the gynecologic department of the Tashkent Regional Oncologic Dispensary were examined. The age of patients ranged from 48 to 77 years. We included 68 women with complaints of bleeding in postmenopause and a history of dysfunctional vaginal bleeding during hormone replacement therapy (HRT) in the study group. The number of patients at the age of menopause was 57 (83.8%) women and 11 (16.1%) cases were at the age of premenopause. The average period of postmenopause was 11 years. Menopause was defined as amenorrhea of at least 12 months' duration in women over 45 years old. All women were examined according to the general scheme: anamnestic data collection, gynecologic examination, risk factor assessment, comprehensive ultrasound examination of pelvic organs, internal organs and retroperitoneal lymph nodes. The most frequent comorbidities in patients were as follpows: hypertension (58.8%), obesity (70.5%), diabetes mellitus (29.4%), thromboembolic diseases (22%) and calculous cholecystitis (14%). Malignant diseases of other localizations: ovarian tumors were registered in 8 (11.7%) patients, breast tumors in 4 (5.8%) women. Malignant diseases in the closest relatives were observed in 3 (4.4%) patients (Tab. 1).
|
3. Results
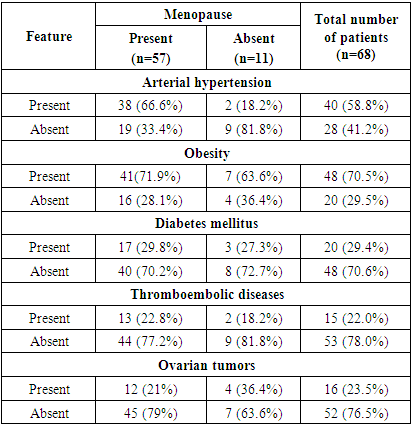
- The presence of adenomyosis was noted in 37 (54.4%) and myoma in 16 (23.5%) women. No significant increase in uterine size was observed in the studied group of patients. In 59 (86.7%) women the uterus was of normal size or with signs of involution. Uterus had smooth clear contours in most cases. In 12 (17.6%) cases the uterine contour was lumpy, which was explained by the subserosal location of myomatous nodules. The uterine cavity was dilated with the presence of anechogenic contents in 8 observations. But this echographic sign is not pathognomonic for endometrial cancer (Fig. 2).
4. Discussion
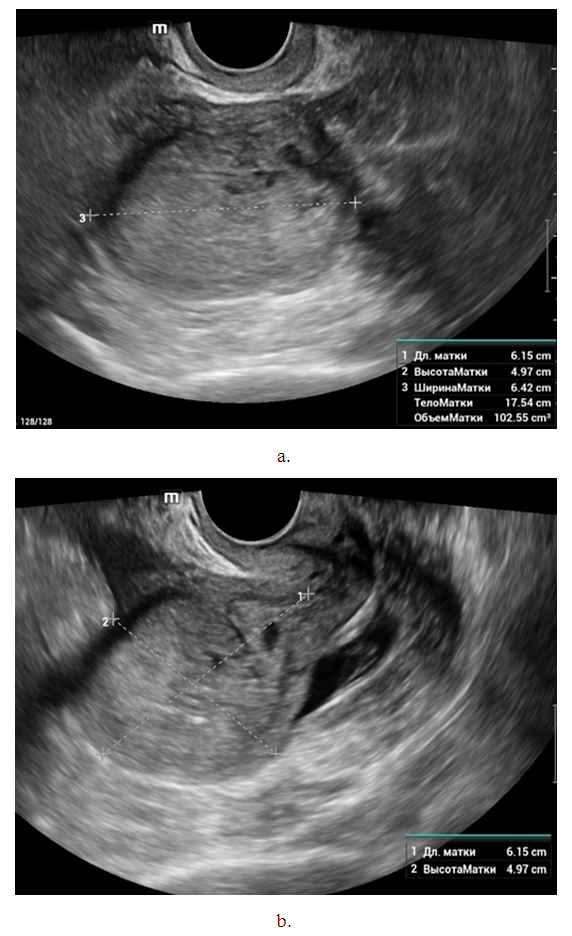
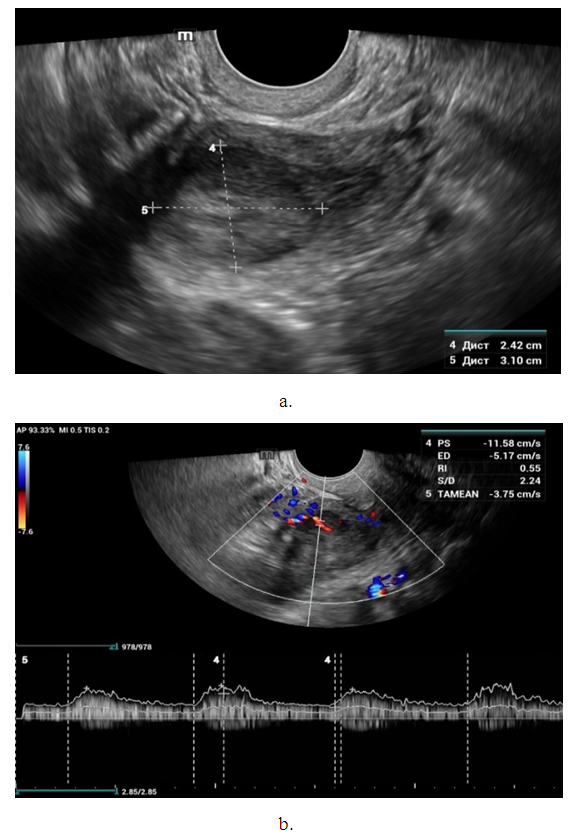
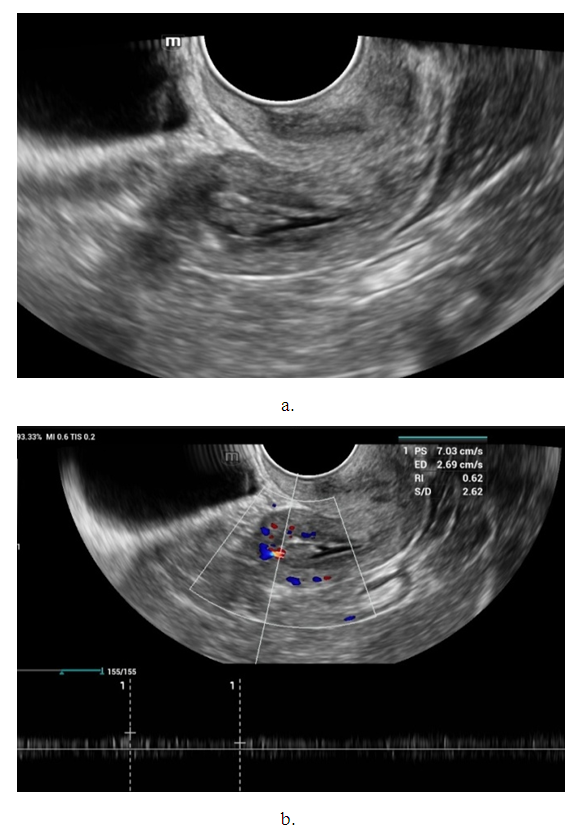
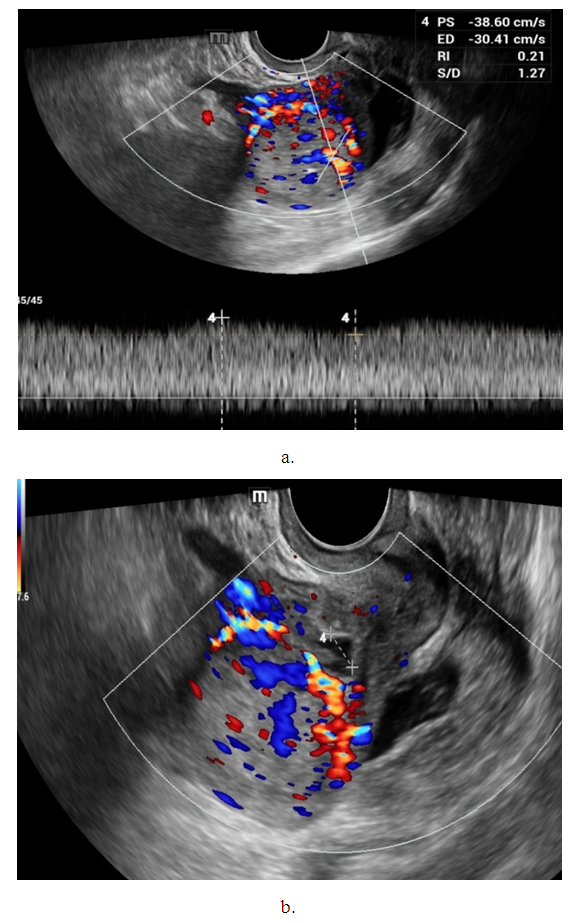
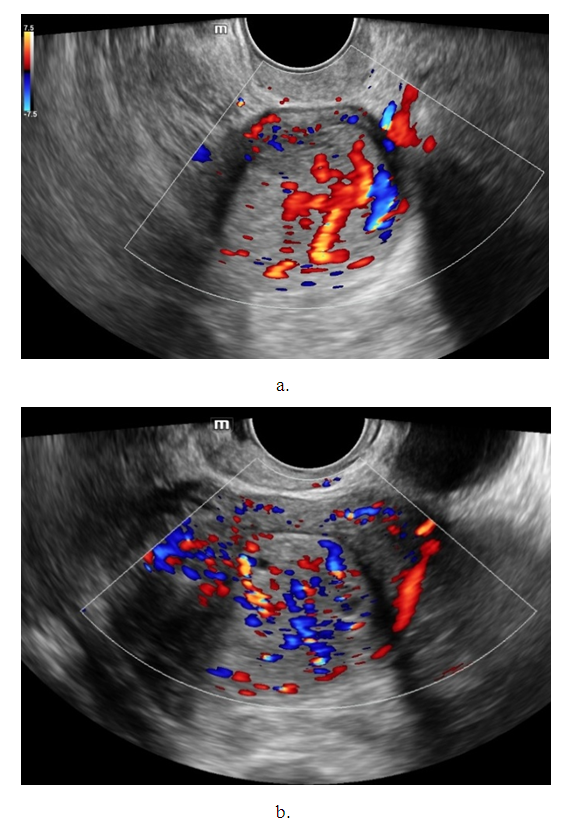
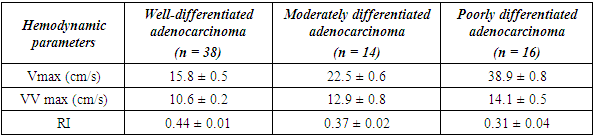
- Noteworthy is the change in the nature of the echo picture of the endometrium depending on its size. According to our observations, when the endometrium thickness was up to 15-18 mm, the echostructure was visualized as hyperechogenic, whereas when the thickness increased to 25-30 mm, it became more heterogeneous due to the visualization of regular and irregular shaped cavity inclusions. It gives the endometrium a heteroechogenic appearance.Thus, it was observed that ultrasonographic features of endometrial cancer such as echostructure and echogenicity varied according to tumor size and stage of the disease. The larger the size of the tumor and the greater the degree of invasion into the myometrium, the more heterogeneous the structure is. There were 10 (14.7%) observations with false negative result due to the presence of microinvasive cancer. Tumor structures in the projection of the uterine cavity were not visualized in this group of patients. We noted diffuse uniform or irregular thickening of M-echo along the entire length of the uterine cavity during transabdominal examination. However, it was not possible to speak more precisely about the localization, tumor shape and degree of invasion. It may be due to the anatomical features of the uterus, the growth pattern of endometrial cancer, and the limited capacity of the transabdominal method. In our opinion, a more promising method for determining the degree of tumor invasion into the myometrium is a comprehensive transvaginal ultrasound examination in the modes of color Doppler scanning (CDS) and elastography. This method is simple, accessible, highly informative, allowing echographic differential diagnostics of various types of uterine oncopathology.An important sign of tumor invasion into myometrium is the definition of endometrial contours and the presence of a hypoechogenic rim defining the border between the tumor and myometrium. On histologic examination, this area was represented by infiltrative changes around glandular elements of highly differentiated cancer. The endometrium had a heterogeneous structure. Thinning of myometrium and infiltrative changes in the form of hypoechogenic rim were observed.The next stage of the study was to perform qualitative and quantitative Doppler imaging. Color mapping mode allowed us to evaluate the presence of blood flow, the localization (peripheral or central) and the number of color loci. Spectral Dopplerometry was performed to measure three main blood flow parameters: arterial (Vmax) and venous flow velocity (VVmax) and the nature of the resistance index (RI) in the uterine arteries and in the tumor area (Fig. 4).
|
5. Conclusions
- Malignant transformation of the endometrium is distinguished by the presence of neovascularization of the neoplasm: intense central and peripheral tumor blood flow, chaotic arrangement of vessels with a low level of peripheral vascular resistance. Ultrasound examination using color Doppler mapping, energy Doppler, and pulsed Dopplerometry does not allow preoperative evaluation of tumor histotype. However, the level of vascularization of the process revealed using color Doppler ultrasonography allows to predict the growth rate of the detected neoplasm.Thus, the conducted study showed that the complex use of multiparametric ultrasound techniques (B-mode, color Doppler imaging, EC) is highly informative and should be used in the differential diagnostics of endometrial cancer in the preoperative period. Joint application of B-mode and Doppler techniques allows to reliably evaluate the heterogeneity of the structure of endometrial tumor process, reliably measure the size of volumetric formations, perform differential diagnostic manipulations and to determine the expediency of surgical intervention. Conflict of interestsThe authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
ACKNOWLEDGMENTS
- The authors express their gratitude to the management of the multidisciplinary clinic of Tashkent Regional Oncologic Dispensary for the material provided for our study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML