-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2022; 10(1): 4-13
doi:10.5923/j.rct.20221001.02
Received: Jun. 13, 2022; Accepted: Jun. 24, 2022; Published: Jul. 15, 2022

Carcinoma Cell-Based Extracellular Matrix Modulates Cancer Cell Communication
Girdhari Rijal , Sienna Becker
Department of Medical Laboratory Sciences, Public Health and Nutrition Sciences, College of Health Sciences and Human Services, Tarleton State University, a Member of Texas A & M University System, Fort Worth, TX 76104, Texas, USA
Correspondence to: Girdhari Rijal , Department of Medical Laboratory Sciences, Public Health and Nutrition Sciences, College of Health Sciences and Human Services, Tarleton State University, a Member of Texas A & M University System, Fort Worth, TX 76104, Texas, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The extracellular matrix (ECM) is a non-cellular dynamic complex forming a 3D spatial network influencing every cell present in the microenvironment. ECM proteins not only provide scaffolding systems for cells, but they also mediate their functions through cell-cell and cell-ECM interactions. Various tissue-mimicking culture systems or 3D tumor models for tumor studies have been advanced using collagen I, Matrigel, ECM and other biomaterials. The ECM secreted by cancer-associated fibroblasts (CAFs) has been studied for its contribution to tumor growth and its significant role in tumor microenvironment, however, ECM deposited by cancer cells has not been studied in detail. This study examined the role of the ECM derived from a TNBC cell line in cancer cell communications. The decellularized ECM of MDA-MB-231 cells has been prepared and used as the cancer ECM (cECM). The cECM hydrogel was prepared and its 3D spatial network was compared to that of collagen I and Matrigel. Cellular activities of MDA-MB-231 cells in cECM, such as proliferation, migration, cell-cell and cell-ECM interactions were compared to collagen I and Matrigel. E-cadherin as a cell-cell interaction marker, and FAK and α3β1 integrin as cell-ECM interaction markers were considered. Their expression by MDA-MB-231 (MM231) cells in different ECMs were subsequently analyzed. Human umbilical vein endothelial cells (HUVECs) were included in this study to observe the role of cECM in their growth since the tumor microenvironment generates many blood vessels. We found that cECM had modulated cell-cell and cell-matrix interactions for the formation of the tumor microenvironment.
Keywords: Extracellular Matrix (ECM), 3D scaffold, TNBC, E-cadherin, FAK, α3β1 integrin
Cite this paper: Girdhari Rijal , Sienna Becker , Carcinoma Cell-Based Extracellular Matrix Modulates Cancer Cell Communication, Research In Cancer and Tumor, Vol. 10 No. 1, 2022, pp. 4-13. doi: 10.5923/j.rct.20221001.02.
Article Outline
1. Introduction
- The extracellular matrix (ECM) is a non-cellular complex part of tissue that provides local microenvironment suitable to the different cells for their optimum functions. Various cells secret both soluble and insoluble (structural) ECM continuously, depending on physio-metabolic needs of an organ [1,2,3,4,5]. Soluble ECM proteins act as factors that define various cell signatures while maintaining tissue integrity, function and development [6,7]. Structural ECM proteins establish scaffolds for cells, providing support for cell adhesion and cell-ECM interactions [1,2,3]. Certain essential fibrous proteins that serve as structural proteins, such as collagen, elastin and laminin, and exhibit concentration differences in various tissues to manifest various functions, such as organ development or disease progression. ECM undergoes deposition, modification and degradation to re-modulate the tissue, making the microenvironment suitable for all cell types within its vicinity [3,8,9,10,11]. ECM with spatial organization and mechanical rigidity facilitates transduction of cell signals for gene transcription, ultimately modifying cell morphology and its activities [12,13]. Certain cell receptors or adhesion molecules (e.g., integrins) are critical for establishing cell-matrix interactions. These trigger mechano-transduction processes to engender the signals necessary for remodulation and regulation of tissue by exhibiting cell proliferation, apoptosis, angiogenesis, and stem cell differentiation [5,8,14]. Therefore, it can be posited that cell-ECM interactions influence tissue homeostasis, wound healing, and overall pathology of diseases. The changes in the ECM lead to disturbance in cell signatures that may contribute to heavy ECM deposition, potentially leading to either fibrosis or tumorigenesis [14,15,16].Our previous study has shown that the collagen percentage is about 90%, with collagen I having the highest concentration in normal breast tissue [2], and that non-collagen proteins, such as glycoprotein and proteoglycan represent the remaining 10%. Laminin and fibronectin are the main glycoproteins, with concentrations in normal breast tissue low compared to collagen types I, III, IV, V and VI [2]. However, deposition of glycoproteins and proteoglycans are higher in cancer tissue compared to normal tissue. Because of the alternation of ECMs, the signature cues in cancer tissue are different from those in normal tissue since most of these proteins directly or indirectly participate in cell-cell and cell-ECM interactions [2].Many studies have pointed out that ECM proteins interact and create the spatial networks which influence cellular activities, increasing expression of certain adhesion proteins and many functional molecules [2,5,14,17,18]. Therefore, our study focused in cancer cell-derived ECM (cECM) for its influences on cell activities, such as cellular proliferation, migration and expression of functional proteins. E-cadherin, focal adhesion kinase (FAK) and α3β1 integrins, which are responsible for communications, were selected for this study since they are responsible for cell behavior, proliferation and migration. E-cadherin is a calcium-dependent cell-cell adhesion protein factor that is essential in epithelial cell behavior and tissue formation with tight cell junctions. Loss of E-cadherin expression leads to loss of contact inhibition thereby supporting epithelial to mesenchymal transformation (EMT), resulting in increased cell motility [19,20,21]. FAK is a tyrosine-phosphorylated protein that is present in cell adhesion sites (focal contacts), promoting interactions with various Src-homology proteins [22,23]. These proteins trigger FAK for downstream processes of ERK2/MAPK, Rac and Rho pathways [24,25,26]. A recent study revealed that FAK-Src signaling is also linked to the regulation of cadherin-mediated cell-cell contacts [27]. The FAK-Rho pathway coordinates the changes in actin and microtubule structure, uniquely controlling the dynamicity of cell adhesion sites and cell motility [28,29]. Integrins are αβ heterodimeric cell surface adhesion receptors for cell-ECM interactions that lead to activation of adhesion-dependent intracellular cell pathways. Integrin-mediated interactions also coordinate other signaling pathways, for example, FAK, Src, Rho, JNK and MAPK molecules [30,31,32]. α3β1 integrin essentially binds to laminins, which is expressed more in epithelial cancer cells [33,34].Collagen I and Matrigel are among the most commonly used ECMs for various cancer studies. Collagen I is a single type of collagen, therefore it does not represent the complex tissue ECMs [35]. However, Matrigel is indeed a complex ECM, it does not represent the microenvironment of human cancer cells since it is derived from the mouse sarcoma, the non-human cancerous tissue [36,37]. It is established that ECM composition influences the cell-ECM and cell-cell interactions, therefore, expression of E-cadherin, FAK and α3β1 integrin in collagen I and Matrigel is not only the same but it differs from human breast cancer tissue. Previous studies have demonstrated that tumor cells also deposit ECM (cECM) besides cancer associated fibroblasts (CAFs), which supports for cancer progression [38,39,40]. The role of cECM deposited in vitro, however, has not been reported in cancer modulation through its support for the expression of E-cadherin, FAK and α3β1 integrin. Therefore, this study uses a hydrogel derived from a triple negative breast cancer (TNBC) cell line, MDA-MB-231 (MM231), together with collagen I hydrogel and Matrigel for cell-cell and cell-ECM interactions. Human umbilical vein endothelial cell (HUVEC) was also included in this study to see the impact of cECM on endothelial cells since there is massive increase in blood vessels in a tumor.
2. Materials and Methods
- Collagen I and Matrigel were procured from Corning, and normal HUVEC and MM231 cancer cell lines were purchased from Promocell and ATCC, respectively. Cell lines were obtained in May, 2020 directly from the providers after cell line characterization. Complete endothelial media (Promocell), DMEM media (Thermo Fisher) and penicillin-streptomycin (p/s, Thermo Fisher) were procured. Both culture media were supplemented with 1% p/s. Primary antibodies targeting collagen I (mouse origin), E-cadherin (rabbit origin), and FAK (mouse origin) were obtained from Novus. Alexa Fluor 488-conjugated α3β1 integrin (rabbit origin) was acquired from Bioss. Alexa Fluor 488-conjugated alpha tubulin antibody was purchased from ThermoFisher. Alexa Fluor 488-conjugated anti-rabbit antibody and Cy5-labeled anti-mouse IgG polyclonal antibody were purchased from Southern Biotechnology and Novus, respectively. CCK-8 reagent and DAPI were procured from Sigma-Aldrich. Green Fluorescent MDA-MB-231 cell line and Green Fluorescent HUVEC were purchased from GenTarget and Neuromics respectively and received by June, 2020.
2.1. Hydrogel Preparation
- Hydrogels from collagen I, Matrigel, and cECM were prepared as previous described [2,41]. Briefly, 2% of collagen I was prepared by adding a pepsin acidic solution. Freeze-dried Matrigel was reconstituted in sterile distilled water at a concentration of 2%. For cECM, MM231 cells were trypsinized and collected after confluent growth in 10 cm culture plates. After centrifugation, the cell pellets were subjected to rapid freeze-thaw cycles for three rounds as previously described [2], and further treated with 0.15% of sodium dodecyl sulfate (SDS) for 3 h, followed by another three rounds of freeze-thaw cycles. Pellets were washed afterwards in phosphate buffer saline (PBS) solution thrice, dried, pasted under liquid nitrogen immersion, and reconstituted in pepsin acidic solution to make the working 2% concentration cECM hydrogel. Hydrogels were kept in a refrigerator at 40C (not more than 2 weeks) and/or in a freezer at -200C (not more than 3 months) until use.
2.2. ECM Coating
- Glass coverslips were first cleaned with 25% nitric acid for 30 min, and rinsed with distilled water (dH2O) for 12 h, changing dH2O every 3 h. They were dried in a safety cabinet overnight and subsequently treated with a 1% silane solution (Sigma-Aldrich) prepared in absolute ethanol for 3 to 5 min, followed by rinsing with absolute ethanol thrice, and with dH2O twice. Finally, the coverslips were air-dried inside the safety cabinet and kept at 4°C in a sealed bag for further use.The coverslips were affixed to the bottom of a 24-well culture plate and kept on ice to prevent the polymerization of hydrogel proteins. Cold samples of 20 μL hydrogels from collagen I, Matrigel, and cECM were poured onto the corresponding coverslip and distributed evenly with a cold applicator. Plates were then placed in an incubator at 37°C for 15-20 min. After complete polymerization of the first ECM coating, coating process was repeated twice allowing cells to migrate downward without touching the coverslip surface.
2.3. Matrix Networks and Porosity
- Upon complete coating of the ECM, the coverslips were washed with PBS, stained with anti-collagen I antibody for 1 h at room temperature, and washed twice with PBS before mounting in Fluoromount (Diagnostic BioSystems). Images were captured using an ECHO Revolve microscope at 100X. The thickness and porosity of the ECM networks were analyzed using ImageJ software (NIH, Public Domain BSD-2).
2.4. Cell Culture on 3D Matrices
- Before seeding the cells in ECM coated coverslips, all the coverslips were thawed at room temperature and washed with 1XPBS twice. Coverslips were dropped into the 24-well culture plate. Five thousand cells/μL of both HUVEC and MM231 cells were added into different wells and cultured using endothelial and DMEM media in 5% CO2 at 37°C, respectively changing media at every alternate day for 14 days. At different time points (1st, 3rd, 7th and 14th day), coverslips were processed for proliferation assay and fixed using 4% paraformaldehyde for immunofluorescence staining (IF) after washing cells with cold PBS twice. Preserved coverslips were stored in a refrigerator at 4°C until staining.
2.5. Proliferation Assay
- The proliferation of HUVEC and MM231 cells on different ECM-coated coverslips was measured with CCK-8 reagent at 1st, 3rd, 7th, and 14th day of culture. Intensity of color developed corresponded to the number of cells. The cells were washed with working PBS twice before addition of the CCK-8 reagent. The reagent was diluted to a 1:10 ratio with PBS and added 200 μL into each well of 24-well plate and incubated at 37°C for 2 h. The absorbance of color developed after 2 h was measured calorimetrically. Furthermore, cells were stained with anti-Ki67 antibody (Novus, rabbit origin) in different time points to monitor the proliferation pattern. Cells were also stained with Alexa Fluor 488-conjugated alpha tubulin antibody and DAPI to demonstrate the proliferative cells at different time points after IF as described below.
2.6. Fluorescent Staining
- At different timepoints (1st, 3rd, 7th and 14th day), culture media was removed and cold PBS was added to the 24-well plates to wash the cells. PBS was replaced by cold 4% formaldehyde to fix the cells. To thaw the cells before staining, 24-well plates were kept at room temperature for 10-20 min and rinsed twice with pre-warmed working PBS. Next, 0.5% Triton X-100 (Sigma-Aldrich) replaced the PBS for the permeabilization of the cells at 37°C for 10 min. Coverslips were rinsed with PBS thrice, followed by three washings with a glycine buffer at pH 7.0 (100 mM glycine in 2 mM HEPES, 2 mM MgCl2, 60 mM PIPES and 10 mM EGTA). Furthermore, to block nonspecific binding sites, a solution of 5% bovine serum albumin (BSA) in glycine buffer was added for 45 min. Diluted primary antibodies in 5% BSA were added to each of the respective wells and incubated overnight at 4°C with constant horizontal rotation of the shaker at a speed of 200 rpm. After rinsing three times with PBS for 5 min, corresponding fluorochrome-labeled antibodies in 5% BSA were added, and the plates were rotated horizontally for 1 h at 200 rpm. Coverslips were rinsed three times with PBS followed by incubation with DAPI (MP Biomedicals) in PBS for 5 min. The coverslips were rinsed three times again with PBS to remove the excess DAPI. The coverslips were then inverted and mounted onto the clean glass slides with the fluorescence mounting media (Fluoromount). Images were captured using ECHO Revolve microscopy under oil immersions. Cell fiber protrusions and expression of E-cadherin, FAK and α3β1 integrin were observed and analyzed using ImageJ software (NIH, Public Domain BSD-2).
2.7. Statistical Analysis
- The statistical data were expressed as means ± SD with one-way analysis of variance (ANOVA) using GraphPad Prism 9.3.0. Error bars represent the SD of the means.
3. Results
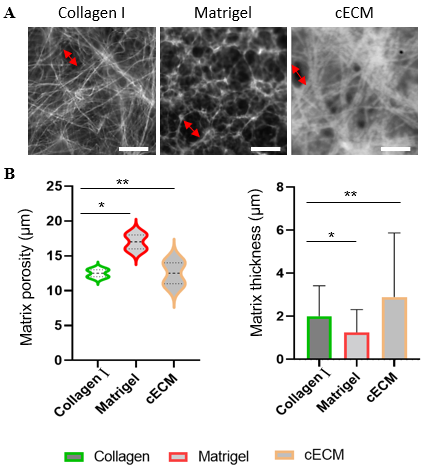
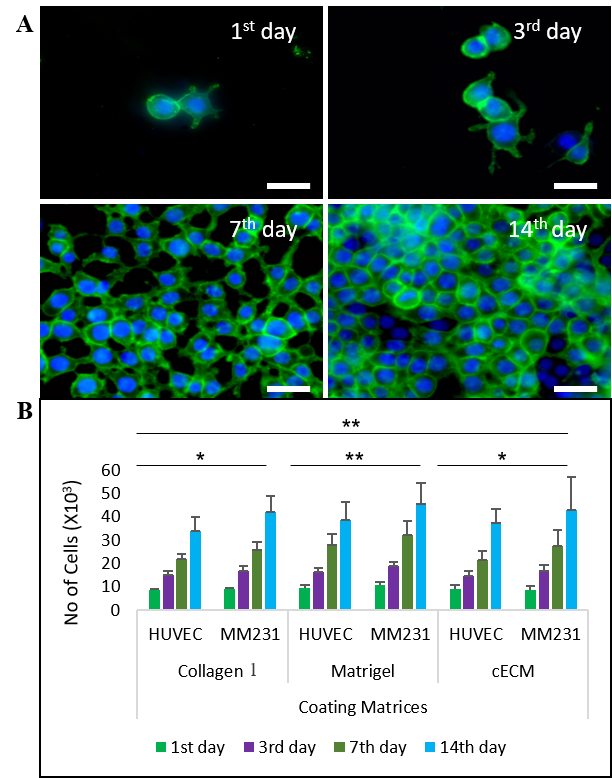
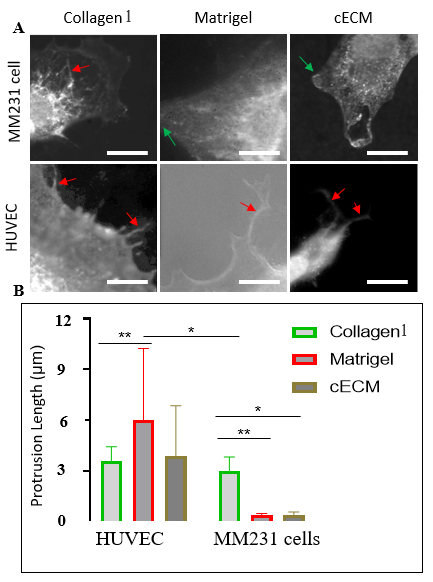
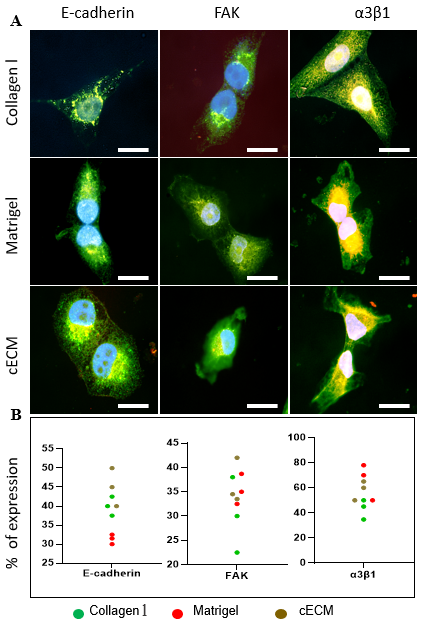
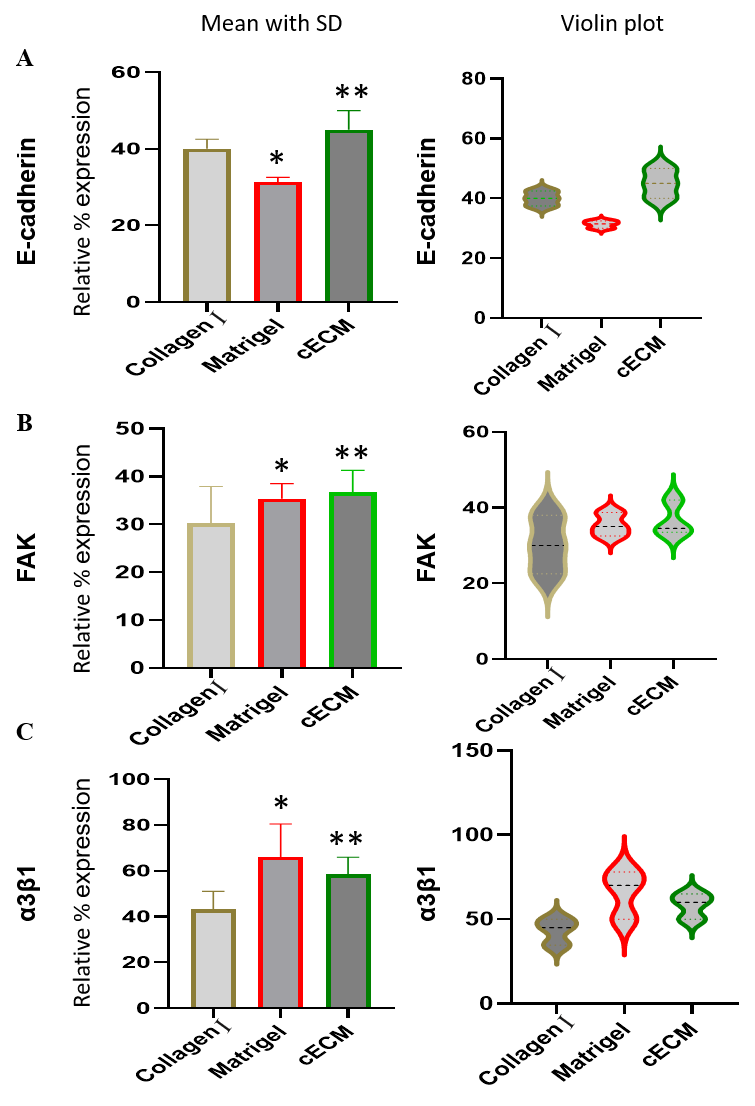
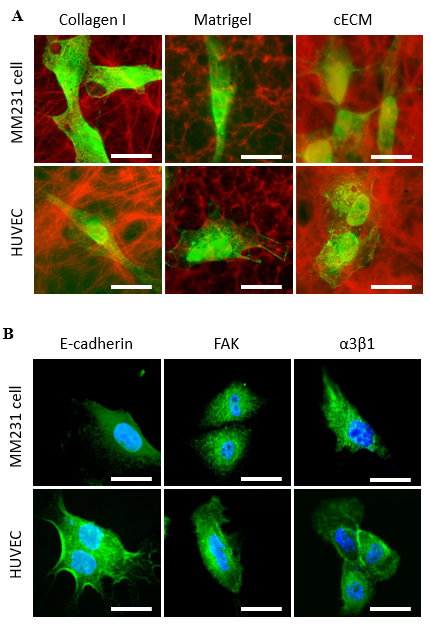
- We used collagen I and Matrigel as controls for cECM, since both are the most commonly used biomaterials for many cancer studies [42,43,44] (Figure 1A). The formed hydrogel ECM network, representing the 3D matrices, allows cells to grow in different spatial directions, including downward [45] (Figures 1A & B). The fibrillar networks in all three different ECMs were demonstrated after staining with collagen antibody (Figure 1A). The cell proliferation in three different ECM networks was demonstrated after analyzed using CCK-8 reagent (Figures 2A & B). Morphology and migration were demonstrated and evaluated after IF of E-cadherin, FAK and α3β1 integrin (Figures 3A-B, 4A-B & 5A-C).
3.1. Morphologies of ECM Fibrillar Networks
- Short fibrils of collagen I and cECM randomly arranged in all directions are shown in Figure 1A. Collagen I formed 3D ECM network of long fibrils and thin bundles in contrast to cECM 3D network which had relatively short and thick bundles (Figure 1A). Matrigel-formed 3D ECM networks of very short fibrils with significantly different arrangement from those of collagen I and cECM (Figure 1A). The ECM configuration establishes the 3D ECM networks having the pores of certain width. The porosity depends on the type of ECM that forms the 3D spatial networks that play the vital role in cell migration and tumor growth. Matrigel had formed the ECM networks with the highest porosity (up to 20 μm) when compared to porosities made of collagen I (up to 15 μm) and cECM (up to 18 μm) (Figure 1B). Consequentially, the structural and constitutional differences of 3D ECM networks of collagen I, Matrigel and cECM establish distinct supporting properties in cells. Therefore, same cells exhibit different characteristics, for example, cell morphology, behavior, proliferation, and molecule expression profiles in different 3D ECM networks.
3.2. Proliferation Assay
- HUVEC and MM231 cells were cultured in the matrix-coated coverslips until cell confluence was reached. The initial growth rate was slow and later it became exponential throughout all matrices (Figure 2A-B). Matrigel supported for more proliferation at the initial phase compared to collagen and cECM. Later, cECM supported more proliferation of MM231 cells compared to collagen I (Figure 2A-B). The growth rate of MM231 was higher than that of HUVEC in 3D ECM networks of collagen I, Matrigel and cECM. The proliferation of cells in cECM network demonstrated that it not only supported for attachment and progression of both HUVEC and MM231 cells, but verified that it participated in the tumor growth.
3.3. Morphology and Migration Patterns
- Morphology of cells cultured in 3D ECM matrices demonstrated that both HUVEC and MM231 cells tend to shrink with relatively smaller sizes compared to their sizes in blank coverslips (without ECM coating) (Suppl Figure 1A & B). An interesting feature that differentiated HUVEC from MM231 cells were the protrusions from HUVEC, which were nearly absent in MM231 cells (Figure 3A-B).The protrusions were more expressed by HUVEC when cultured in collagen I than in Matrigel and cECM. However, relatively longer with a few numbers of protrusions seen in HUVEC cells in both Matrigel and cECM indicates that they support for cell migration with less cell expansion. The cell size, however, was relatively smaller in cECM with less migration than in Matrigel and collagen I (Suppl Figure 1A). Interestingly, intracytoplasmic protrusion-like fibers were demonstrated within some MM231 cells when grown in collagen I, but they were not observed in cells grown in Matrigel and cECM (Figure 3A). It is still not clear whether the invasion rate is affected by the smaller or larger and external or internal fiber-like projections.
3.4. E-cadherin Expression
- E-cadherin expression was significantly weaker in MM231 cells when cultured in blank coverslips (Suppl Figure 1B), showing the consistent results of previous studies [46]. However, E-cadherin expression in MM231 cells was upregulated when cultured in matrices. Higher E-cadherin expression in cells during 3D cultures indicated that matrices supported for cell-cell interactions [46,47]. Interestingly, the higher expression of E-cadherin in MM231 cells was observed when cultured in cECM than in collagen I and Matrigel matrices (Figures 4A-B & Figure 5A). This might be the reason of smaller MM231 cells in cECM than in collagen I and Matrigel. Increasing in cell-cell interactions reduces the intercellular space making the compact arrangement of the cells that help to reduce the overall cell size [48]. The E-cadherin expression pattern in HUVEC was however seen in blank and in all three different ECM networks without significant changes (Suppl Fig 1B).
3.5. FAK Expression
- Similar to E-cadherin expression, more FAK expression in MM231 cells was observed in cECM compared to its expression in collagen I and Matrigel (Figure 4A-B and Figure 5B). More FAK expression supported by cECM indicates that it increases cellular dynamicity and motility more than by collagen I and Matrigel. The FAK expression pattern by HUVEC was, however, not significant different from its expression by MM231 in different ECM networks.
3.6. α3β1 Integrin Expression
- In contrast to E-cadherin and FAK expression patterns, α3β1 integrin expression in MM231 cells was weaker in cECM than in Matrigel (Figure 4A-B and Figure 5C). However, MM231 cells expressed more α3β1 integrin in cECM than in collagen I, indicating that cECM participates more in α3β1 integrin related signaling pathways compared to collagen I. α3β1 integrin expression pattern in HUVEC was similar with MM231 cells in different ECM networks.
4. Discussion
- Understanding the tumor cell microenvironment is imperative due to its critical role in tumor growth and metastasis. The microenvironment guides cell-cell and cell-matrix interactions for various cell functions, such as proliferation, differentiation, survival and migration [49]. Many studies have shown that ECM plays a key role in maintaining the tissue microenvironment [1,2,8,9,15]. In light of this, cECM was included in this study together with collagen I and Matrigel for the formation of 3D ECM networks to reveal and compare its contribution to cell proliferation, migration, and cell-ECM interactions. The distinct nature of the fibers made of cECM in thickness, width and network in 3D has a unique impact in cell morphology, proliferation, attachment, and expression of factors required for cell-cell and cell-ECM interactions [1,49,50]. This study discovered that cECM not only supported cell proliferation, but also facilitated cellular communications. The proliferation rate was slower at first in cECM compared to collagen I and Matrigel, but the rate of proliferation showed a greater increase in cECM than in collagen I (Figure 2A-B). Previous studies have shown that ECM spatial networks support fiber protrusions in cells [51,52]. Related to this, we found that HUVEC had many fiber protrusions when grown in collagen I, and relatively longer protrusions when grown in Matrigel (Figure 3A-B). The longer fiber protrusions were also observed in HUVEC in a few numbers when grown in cECM. Fiber-like external protrusions were not clearly observed in MM231 cells, however, intracytoplasmic protrusion-like fibers were observed when cultured in collagen I (Figure 3A). The extended cytoplasmic wide ends were observed in MM231 cells when grown in Matrigel and cECM (Figure 3A-B). The results were consistent with the previous studies that external ECMs influence the cell ECM alignments for the fiber protrusions or for the extended ends [51,53]. Furthermore, it is found that adherens junctions participate in cellular adhesion by both cell-cell and cell-matrix interactions, controlling proliferation and cell polarization that are crucial to maintain tissue homeostasis [54,55]. The reduction in cellular adhesion has been correlated with more aggressive cell migration, influencing tissue integrity [50,56]. Fiber protrusions support for the formation of many cell-ECM junctions and guide for the direction of cellular migration (Figure 3A-B) [57,58]. Some studies have revealed that a high-density of ECM downregulates E-cadherin expression supporting cell migration [59]. It is a true for the cells that express E-cadherin more in blank (without ECM coating) than in ECM 3D culture. But, in our study, we particularly focused in MM231 cells that did not express E-cadherin in blank (Suppl fig 1B), but expressed it in 3D ECM networks (Figure 4A-B) [46,47]. The regulation of E-cadherin plays a vital role in enhancing cell-cell junction for tissue integrity. Down regulation of E-cadherin usually leads to a loss of cell polarity, resulting in increased proliferation and migration. The expression of E-cadherin in ECM (collagen I, Matrigel and cECM) demonstrated that MM231 cells exhibit its expression in their tissue (Figure 4A-B and Figure 5A) [60,61]. There is a controversial opinion about the role of E-cadherin since its high expression either suppresses or promotes tumor progression and regulation of EMT, as well as other factors [62,63]. E-cadherin controls tumor metastasis at multiple points as a tumor suppressor protein, and its loss of expression leads to EMT [62]. Some cancers, however, express E-cadherin continuously with the EMT phenomenon. Therefore, E-cadherin expression and its role depends on environmental factors and cancer types [62,63]. In this study, MM231 cells expressed more E-cadherin in cECM than in collagen I and Matrigel (Figure 4A-B and Figure 5). Its less expression in collagen I may be due to a single type ECM in collagen I that does not influence cell-cell interactions, supporting for the cell migration because of its thicker and longer fibers than in Matrigel 3D ECM made of thinner and shorter fibers (Figure 1A-D and Figure 4A-B). In general, a high ECM concentration increases the mesenchymal phenotypes of cells and enhances cell-ECM interactions, but impairs the cell-cell interactions [8,64]. The high density of collagen I increases the integrin-mediated cell-ECM interactions that are seen regulating cell proliferation and migration via phosphorylated FAK and ERK signaling [65,66]. FAK, a non-receptor tyrosine kinase, plays a key role in various cellular processes, like cell-ECM adhesion, proliferation, and migration. Recent studies have shown that FAK also participates in cell-cell interactions since suppression of cell-cell interactions by inhibiting FAK leads to dysregulation of cell morphology [67,68]. Expression of FAK has been shown by MM231 cells in collagen I, Matrigel and cECM, but higher expression was shown in cECM (Figure 4A-B and Figure 5B). Many studies have demonstrated that FAK performs a dual role in promoting cell motility and invasion through the activation of distinct signaling pathways, for example, downstream regulation of Ras/Erk1/2 and PI3k/Akt, and upstream regulation of integrin/FAK, cadherin/Stat3 and Rho [68,69]. Higher expression of FAK in cECM is an indicator that cells are more invasive nature while grown in cECM since it had been demonstrated that FAK facilitated cellular stiffness and contractile force for the invasion of dense 3D extracellular matrices [70,71].Many types of cells express α3β1 integrin, which mediates cellular adhesion to the matrix ligand laminin-5 and promotes tumor growth by increasing cell-ECM adhesion, proliferation, migration, and invasion. Contrastingly, α3β1 integrin may have anti-metastatic activity in some conditions [72]. The α3β1 integrin regulates tumor-host interactions within the metastatic tumor microenvironment to limit tumor growth [72]. The expression of α3β1 integrin was higher in MM231 cells when grown in Matrigel than in collagen I and cECM (Figure 4A-B and Figure 5C). Its expression more in cECM than in collagen I demonstrated that cECM provided better focal adhesion for cell proliferation and migration of MM231 cells than collagen I. Another study has shown that α3β1 also regulates cell-cell contact through distinct protein interaction sites within its β-propeller [72]. Integrin α3-silenced cells show increased proliferation because of upregulation and nuclear localization of cyclin-dependent kinases with the support of activated AKT and ERK. However, the increase in cell migration is found to be independent of AKT or ERK pathways [73]. Additionally, receptors for nuclear factor-kβ, Bcl-2 and epidermal growth factor, which are positively correlated with cell proliferation and survival, have increased their expressions in integrin α3-silenced cells. Overall, integrin α3β1 plays key roles in regulating cell proliferation and migration [74].
5. Conclusions
- This study highlights the perspectives of interest on cancer progression through the cECM, an important protein complex secreted by cancer cells within their microenvironment next to massive ECM deposits from CAFs during cancer growth. Expanded studies on the many cECM proteins secreted by cancer cells are necessary to decipher the fundamental processes of cancer initiation and growth through 3D culture systems. This study’s observations may be helpful in identifying targets for therapeutic avenues to impair interactions between cell-to-cell and cell-to-ECM, therefore controlling cancer growth.
ACKNOWLEDGEMENTS
- The authors thank the colleagues and students of the Medical Laboratory Sciences, Public Health and Nutritional Sciences, TSU for their critical comments and suggestions.
Funding
- This work was supported by Tarleton State University summer research fund (2020) and President’s Excellence in Research Scholars (PERS) (2021) provided to Girdhari Rijal.
Ethic Declarations
- Not applicable
Consent for Publication
- No applicable
Conflicts of Interest
- The authors declare that they have no competing interests. Parts of its data have been presented in Tarleton State University’s PERS Symposium held on 9th Feb 2022.
Supplementary Material
- Suppl figure 1
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML