-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2020; 8(1): 1-7
doi:10.5923/j.rct.20200801.01

Leaf Lipid Chromatographic Fractions of Annona muricata and Their Effects on Indices of Prostate Cancer
Eneh F. U.1, Eneanya S. U.1, Eneh C. I.2, Ezekwesili C. N.1, Okonkwo K. O.1, Eneh C. J.1, Nwoke C. H.1, Anijah S.1, Moses E.1
1Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Nigeria
2Department of Paediatrics, College of Medicine, Enugu State University Teaching Hospital, Enugu, Nigeria
Correspondence to: Eneh C. I., Department of Paediatrics, College of Medicine, Enugu State University Teaching Hospital, Enugu, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Prostate cancer and other prostatic diseases have been proven to be the major cause of death in men between the ages of 60 to 90 years across the globe. Recently, various plant parts are being explored for their potential in disease management. Soursop is a medicinal plant used for the treatment of various diseases in animals and man, most recently cancer. This study investigated the varied effects of three different fractions (200 mg/Kg intraperitoneal, days 1 to 5) of soursop (Annona muricata) leaf lipid fractions on the Prostate Specific Antigen (PSA) and Prostatic Acid Phophatase (PACP) levels of healthy male albino rats. The leaf lipid was extracted using soxhlet extractor from dried A. muricata leaves. Further purification was achieved using column chromatography. Thirty healthy male albino rats were randomly separated into five groups; baseline, control, test A (simple lipid treated group), test B (complex lipid treated group) and test C (glycolipid treated group), n=6. The animals were sacrificed on the sixth day. Blood samples were obtained via intracardiac puncture and analyzed for PSA and PACP. The simple lipid fraction of A. muricata leaf extract showed to be the most potent but extremely toxic at the used concentration as there was significant decrease in PACP and total PSA with accompanied decrease in free PSA and 83.3% mortality rate in test A group in comparison to the baseline and control groups. The complex lipid fraction on the other hand was fairly potent with 33.3% mortality rate while the glycolipid fractions showed rather negative results than potency. The findings from this study suggest that the simple and complex lipid chromatographic fractions of A. muricata leaf extract have the potentials for prostate cancer management.
Keywords: Prostate, Cancer, Diseases, Soursop, Prostate Specific Antigen, Acid Phosphatase, Soxhlet, Chromatography, Anonna muricata, Complex lipids, Simple lipids, Glycolipids
Cite this paper: Eneh F. U., Eneanya S. U., Eneh C. I., Ezekwesili C. N., Okonkwo K. O., Eneh C. J., Nwoke C. H., Anijah S., Moses E., Leaf Lipid Chromatographic Fractions of Annona muricata and Their Effects on Indices of Prostate Cancer, Research In Cancer and Tumor, Vol. 8 No. 1, 2020, pp. 1-7. doi: 10.5923/j.rct.20200801.01.
Article Outline
1. Introduction
- Several life-threatening diseases have been waging war against humanity in the last few decades, most likely as a result of the changes in the nature of man’s environment and inadequate nutrition/unhealthy life styles. The most prominent of these environmental factors is industrial waste (gaseous, liquid and solid), which does not only contaminate the water we drink, the food we eat and threatens aquatic life but also depletes the ozone layer thereby increasing our susceptibility to various diseases caused by radiation of which cancer is the chief. Cancer is a disease capable of attacking almost all parts of human body as it involves the malfunctioning of the cells (Mishra et al., 2013; Owolabi et al., 2013). There are several types of cancer which include; breast cancer, glioma, leukemia, prostate cancer, cervical cancer, cancer of the lungs, cancer of the liveramong many others.Cancer results from uncontrolled proliferation of cells due to failures of growth control mechanisms (Osorio et al., 2007; Baade et al., 2009; Mohamad et al., 2015). The most common type of cancer in men is prostate cancer (Asareet al., 2014; Mottent et al., 2015). The record of Prostate Cancer Foundations (2005-2012) has it that one out of six men would have prostate cancer at some point in their lifetime. Prostate cancer is a type of cancer that affects the prostate gland of the male reproductive organ and is more often than not, diagnosed after the age of 40 years (Mohamad et al., 2015). It is prevalent in older men and has been proven to be the major cause of death in men between the ages of 60 to 90 years (Torres et al., 2012). Heredity and increasing age has been identified as risk factors for prostate cancer with heredity presenting an onset which is usually six to seven years earlier than spontaneous cases (Mottent et al., 2015). Being the most frequently reported cancer in male, this increases economic burden significantly across the globe. There are no data presently suggesting any medical intervention for the successful treatment of prostate cancer. Several pharmaceutical formulations such as 5-alpha-reductase inhibitors and α-adrenoceptor antagonists have not been effective in terminating the progression of prostate cancer (Mottent et al., 2015).There are various biological biomarkers in the body, used for disease prognosis. Most of these markers are enzyme which are normally found at reduced level in the plasma but develops increased activity in pathological states. Prostatic Acid phosphatase (PACP) and prostate specific antigen (PSA) are prominent indices for prostate cancer (Veeramani et al., 2005; Menez et al., 2008). PSA is a serine protease secreted exclusively by prostatic secretion epithelium and it contributes to semen liquefaction through the hydrolysis of semenogelin (Iweala et al., 2015). PSA levels higher than 2 ng/mL is considered a high risk of metastasis and death after some years (Mottent et al., 2015). Acid phosphatase is an enzyme involved in the hydrolysis of inorganic phosphomonoesterases in acidic medium (Agbafo, 2015). Both have their various and unique functions at adequate level in the prostate gland and the whole body at large.Several plants are being investigated for the treatment of various diseases as medicinal plants are used globally for the preservation of health (Coria-Te´llez et al., 2016). A. muricata has so far been explored for its indication in the management of diseases such as inflammation, insomnia, diabetes, rheumatism, infections, arthritis, cancer among others (Boyom et al., 2011; Owolabi et al., 2013; Ezuruike and Prieto, 2014; Gavamukulya et al., 2014; Ishola et al., 2014; Moghadamtousi et al., 2015; Yang et al., 2015). Annona muricata, which is commonly called soursop or graviola is specie of the genus Annona of the custard apple tree family, Annonaceae, a native to Amazon rainforest (Coria-Te´llezet al., 2016). It is a small tree growing up 5 metres (15ft) tall, whose bark, leaves, roots, seeds and fruits are presently being used as traditional remedies in South America, Asia and other countries (Pinto et al., 2005). The leaf and seed are the most therapeutic parts of the tree probably due to the high content of phytochemicals in them. A. muricata leaf contains several phytochemicals which include: Annonaceousacetogenins, essential oils, alkaloids, phenols among others (Kossouohet al., 2007; Gajalakshmi et al., 2012; Mishra et al., 2013; Coria-Te´llezet al., 2016).Although independent studies have shown the different plant parts of A. muricata to be potent against various diseases and infections (Boyom et al., 2011; Roslida et al., 2012; Vijayameena et al., 2013; Ezuruike and Prieto, 2014). Similarly, it has been shown that the leaf extract of A. muricata is effective against tumour and cancer cells (Torres et al., 2012; Mishra et al., 2013; Owolabi et al., 2013; Paul et al., 2013; Asare et al., 2014; Gavamukulya et al., 2014; Pieme et al., 2014; ). Further research has also shown that acetogenin fraction is very effective but highly toxic while suggesting that crude A. muricata is somewhat safer with good therapeutic effect because of the synergistic reaction between the acetogenins and the flavonoid (Yang et al., 2016). Comprehensive studies have equally been done on the toxicity of A. muricata (Coria-Te´llez et al., 2016). More so, Thang et al. (2012) studied the volatile oil component of soursop leaf extract. Amidst all these studies, there is no sufficient information on the different lipid components of this leaf extract and their varied efficacy/toxicity concerning prostate cancer. However, this study was designed to investigate the impact of the administration of different fractions of A. muricata on the indices of prostate cancer the the quest to identifying the fraction with the highest potential efficacy with accompanied minimum toxicity.
2. Methodology
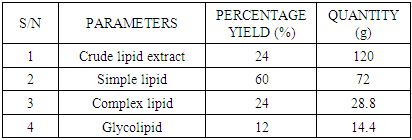
- SAMPLE COLLECTION AND PREPARATIONA. muricatafresh leaves were collected at Umuru village, Ogidi in Idemili North Local Government Area, Anambra state and were identified in Botany department, NnamdiAzikiwe University, Awka, Nigeria, by Dr. Ogbuozobe. The fresh leaves were washed with distilled water and air dried for ten days at room temperature and ground with clean manual grinder to obtain its powder.METHOD OF LEAF LIPID EXTRACTIONTotal of five hundred grams (500g) of the powdered leaf was subjected to soxhlet extraction; twenty grams (weighed and rapped with filter paper) per extraction for sixteen (16) hours using 300 ml of N-Hexane for each batch of extraction. After each extraction, the samples were removed from the extractor and the hexane used for the extraction recovered leaving the lipid extract which was then placed in the water bath for removal of the residual solvent. The weight of the lipid extract and the percentage yield were then calculated.Principle of Soxhlet ExtractionHeat from the heating mantle heats up the flask until the boiling point of the solvent in the flask is reached. The boiling solvent vaporizes due to constant heat supply and tends to evaporate through the condenser. The condenser (containing water at room temperature), converts the gas into a liquid that trickles into the extraction chamber containing the sample. The solvent continues to drop on the sample until its level becomes even with the apex of the siphon arm. Reflux occurs at this point thereby washing down extracts from the sample into the flask.FRACTIONATION OF LEAF LIPID EXTRACTThe leaf lipid extract was purified and/or separated into three different classes of lipid using column chromatographic technique. A short wide column (4 cm diameter and 15 cm length with a glass frit and tap) was carefully packed with hundred grams (100g) of silica gel, prepared with n-Hexane using slurry method. The solvent was allowed to drain until it became even with the surface of the stationary phase. The sample was then introduced carefully using pipette (3g/100g adsorbent) and allowed for few minutes to be absorbed into the gel. Chloroform, 10 column volumes was used to elute simple lipid. Acetone, 10 column volumes was used to elute complex lipids. Methanol, 10 column volumes was used to elute glycolipids in that order. The percentage yields were calculated. The principle is based on adsorption, partition, affinity and gravity.PREPARATION OF STOCK SOLUTIONThe stock solution for each of the test groups was prepared using the sum of the body weights of the experimental animals in each group receiving the lipid fractions with reference to 200 mg/kg body weight concentration. This was made up to 10 ml using 80% tween 80 (which served as a placebo/vehicle). The stock for the control groups contained 10 ml of 80% tween 80 only.EXPERIMENTAL ANIMALSThirty healthy male albino rats were purchased from Chris Farm, Awka, Anambra state. They were divided into five groups; the baseline, control, test A, test B, and test C, (n=6) and were put in five separate cages. The animals in each group were tagged by labeling with a specific color of permanent marker. The animals were kept and fed for seven days period of acclimatization.WEIGHING OF ANIMALSThe experimental animals were weighed on the day of purchase, tagged and their various weight recorded in grams. They were also weighed before being sacrificed to obtain their final weight after extract administration.ADMINISTRATION OF LEAF LIPID FRACTIONThe leaf lipid fractions were administered to the rats in the different test groups as specified by the model via intraperitoneal route for five days. The control group received 80% tween 80 only via intraperitoneal route for five days. The quantity in milliliter is based on their body weights with reference to 200 mg/kg body weight. Test A group received simple lipid fraction while test B group received complex lipid fraction and test C group was treated with glycolipid fraction.SACRIFICE OF ANIMALS AND BLOOD SAMPLE COLLECTIONThe rats were mildly anaesthetized (using chloroform) and a cardiac puncture was performed on the unconscious rats to obtain blood samples which were collected slowly. The blood samples collected were carefully transferred to centrifuge plastic tubes to avoid haemolysis of the blood and then left standing for some minutes to clot. The clotted blood was centrifuged at 3000 r.p.m for 30 minutes, the sera were collected and transferred into labeled plain specimen bottles. The individual serum was then analyzed forTotal Prostate Specific Antigen (TPSA), Free Prostate Specific Antigen (FPSA), Non-prostatic Acid Phosphatase (NPACP) andTotal Acid Phosphatase (TACP).PROCEDURE FOR PROSTATE SPECIFIC ANTIGEN DETERMINATIONThis is an immunoenzymatic assay that requires antibodies with affinity, avidity and specificity with different and distinct epitope recognition in excess and native antigen. The prostate specific antigen (PSA) ELISA test is based on the principle of a solid phase enzyme-linked immunosorbent assay. The assay system utilizes a goat anti-PSA directed against PSA for solid phase immobilization (on the microliter wells).The test sample is allowed to react first with the immobilized antibody at room temperature. The wells were washed to remove any unbound antigen. The monoclonal conjugate was then added and allowed to react with the immobilized antigen, resulting in PSA molecule being sandwiched between the solid phase and enzyme-linked antibodies. The wells were washed with water to remove unbound antibodies. A solution of tetramethylbenzidine (TMB) reagent was added and incubated. This resulting in the development of blue color which was later stopped with the addition of stop solution (1N HCl) changing the color to yellow. The concentration of PSA is directly proportional to the color intensity of the test sample. The absorbance was measured spectrophotometrically at 450 nm.For Free Prostate Specific Antigen (FPSA) determination, immobilization occurs during the assay at the surface of microplate well through the interaction of streptavidin coated on the well and exogenously added biotinylated monoclonal (anti-PSA) antibody. Upon mixing monoclonal biotinylated antibody, the enzyme labeled antibody and a serum containing the native antigen, reaction resulted between the native antigen and the antibodies, without competition or steric hindrance to form a soluble sandwich complex. The interaction can be illustrated by the following equations:EnzAb(p) + AgFPSA + BtnAb(m) ka↔k-aEnzAb(p)- AgFPSA-BtnAb(m)BtnAb(m) = Biotinylated antibody (excess quantity)AgFPSA = Native antigen (variable quantity)EnzAb(p) = Enzyme labeled antibody (excess quantity)EnzAb(p)- AgFPSA-BtnAb(m) = Antigen-Antibodies complexKa= rate constant of associationk-a = rate constant of dissociationSimultaneously, the complex is deposited into the well through the high affinity reaction of streptavidin and biotinylated antibody. The interaction is shown below:
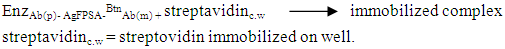 After equilibrium has been attained, the antibody-bound fraction was separated from unbound antigen by decantation or aspiration. The enzyme in the antibody bound fraction is directly proportional to the native antigen concentration.PROCEDURE FOR ACID PHOSPHATASE DETERMINATIONAcid phosphatase catalyses the hydrolysis of α-Naphthylphosphate.
After equilibrium has been attained, the antibody-bound fraction was separated from unbound antigen by decantation or aspiration. The enzyme in the antibody bound fraction is directly proportional to the native antigen concentration.PROCEDURE FOR ACID PHOSPHATASE DETERMINATIONAcid phosphatase catalyses the hydrolysis of α-Naphthylphosphate. The α-Naphthol released from the substrate by acid phosphatase is coupled with Fast Red TR (diazotized 2-amino-5-chlorotoluene) to produce a colored complex that absorbs light at 405 nm. L-Tartrate on the other hand inhibits prostatic acid phosphatase but does not interfere with the reaction mechanism. Therefore, if testing is performed in the presence or absence of L-Tartrate, the difference between the results of the two assays is the level of prostatic acid phosphatase in the serum. Citrate buffer, substrate, tartrate (for non-prostatic) and serum were added to appropriately labeled test tubes before 1hour incubation at room temperature. Hydrolysis was stopped using sodium hydroxide. Sodium bicarbonate, 4-Amino-antipyrine and Potassium ferricyanide were added and mixed properly. A color change was observed and the intensity of the red color was measured by spectrophotometer at 405 nm.STATISTICAL ANALYSISThe Graphpad Prism 6 software was used to determine the statistical significance within the different experimental groups. The test of significance was applied and values obtained as p<0.05 were considered as statistically significant. The data generated were expressed as mean ± SD. Student t-test was used followed by Holm-Sidak’s multiple comparison tests.
The α-Naphthol released from the substrate by acid phosphatase is coupled with Fast Red TR (diazotized 2-amino-5-chlorotoluene) to produce a colored complex that absorbs light at 405 nm. L-Tartrate on the other hand inhibits prostatic acid phosphatase but does not interfere with the reaction mechanism. Therefore, if testing is performed in the presence or absence of L-Tartrate, the difference between the results of the two assays is the level of prostatic acid phosphatase in the serum. Citrate buffer, substrate, tartrate (for non-prostatic) and serum were added to appropriately labeled test tubes before 1hour incubation at room temperature. Hydrolysis was stopped using sodium hydroxide. Sodium bicarbonate, 4-Amino-antipyrine and Potassium ferricyanide were added and mixed properly. A color change was observed and the intensity of the red color was measured by spectrophotometer at 405 nm.STATISTICAL ANALYSISThe Graphpad Prism 6 software was used to determine the statistical significance within the different experimental groups. The test of significance was applied and values obtained as p<0.05 were considered as statistically significant. The data generated were expressed as mean ± SD. Student t-test was used followed by Holm-Sidak’s multiple comparison tests.3. Results
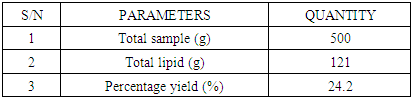
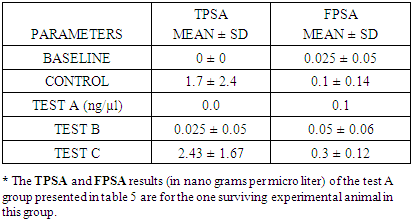
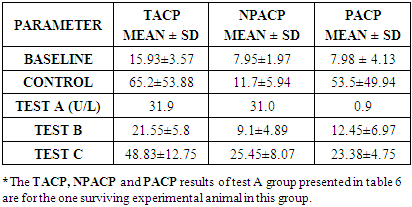
- The total percentage yield of Annonnamuricata leaf n-hexane extract was 24.4% (121 g) of crude lipid was obtained from 500 g of mashed A. muricata leaf n-hexane extract.
|
|
|
|
|
|
4. Discussions
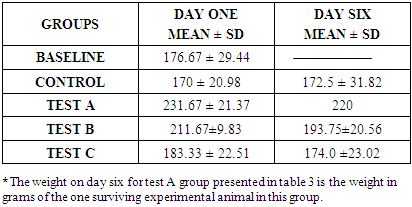
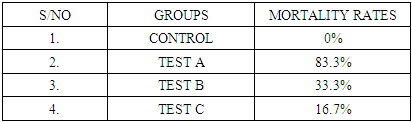
- In the last few decades, A. muricata has been explored for its indication for the treatment of several diseases and infections (Boyom et al., 2011; Owolabi et al., 2013; Ezuruike and Prieto, 2014; Moghadamtousi et al., 2015). Extracts from various parts of A. muricata have reportedly displayed selective cytotoxic effects against various cancer cell lines (George and Kumar, 2012; Torres et al., 2012; Paul et al., 2013, Mohamad et al., 2015). In addition, it has been reported that the n-hexane extract inhibited cell proliferation in 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT assay) and was believed to have triggered cell death in Capan-1 cells probably via necrosis and/or apoptosis (Mohamad et al., 2015). Studies have equally shown that n-hexane extract of A. muricata leaves have the most effective inhibition of cell proliferation (Mohamad et al., 2015). Torres et al. (2012) showed that A. muricata extracts inhibited the phosphorylation of the major molecules implicated in the extracellular signal-regulated kinase (ERK) and the phosphatidylinositol 3’kinase (PI3 K/ Akt) pathway which is important for the proliferation and survival of pancreatic cancer cells. This potency (especially with respect to prostate cancer) and toxicity attributed to soursop plant led to this chromatographic study. This study investigated the effects of three distinct fractions of A. muricata leaf extract on prostate cancer indices. The result obtained in this study indicated that the simple lipid fraction of Annona muricata leaf extract may be very potent against prostate cancer but highly toxic at the used concentration. The simple lipid fraction was shown to significantly increase the free PSA and satisfactorily decrease (zero) the total PSA of the test A group when compared to vehicle treated control and baseline groups. Similarly, it also significantly decreased the prostatic ACP of the test A groups when compared to treated control and baseline groups. The result of the simple lipid treatment is similar to the findings of Yang et al. (2015) among others, who showed that acetogenins are the most selective cytotoxic component of A. muricata as the acetogenin-enriched fraction was the most effective inhibitor of proliferation in human prostate cancer PC-3 cells in their investigation. Their study also indicated that the acetogenin fraction is more potent against prostate cancer than the crude extract. This is suggestive of more sophisticated purification & dose adjustment to arrive at the desired result. The cytotoxicity mechanism of Acetogenin has been postulated to involve apoptosis through the inhibition of the mitochondrial complex I, and the ubiquinone-linked NADH oxidase in cancer cell'splasma membranes (Pieme et al., 2014).However, we recorded high mortality rate (83.3%). Expectedly, the surviving test animal in this group showed other side effects such as loss of appetite, drastic weight loss, loss of hairs majorly on the abdomen and upper stomach. The potency/toxicity and high mortality may be attributed to the concentration used and/or the presence of waxy toxic A. acetogenins which is possibly eluted with simple lipids as waxes are also component of simple lipids. Sequel to the vast number of A. muricata preliminary anticancer research, it was discovered that the major bioactive components known as annonaceous acetogenins (ACGs) are the major contributor to anticancer effect (Mohamad et al., 2015). The observed toxicity is in support of the findings of Yang et al., (2016) which claimed that the separation of phytochemicals in other to isolate the most active component produces toxicity. They further emphasized that the isolation of acetogenin, despite its superior efficacy gave rise to toxicity in mice. Other components of the oil such as (E)-carophyllene, δ-cadiene, α-humulene and phenylpropanoid eugenol have also been found to be cytotoxic (Owalabi et al., 2013). The complex lipid fraction of A. muricata equally showed moderate therapeutic effect and at the same time reduced side effect on the test animals. This fraction significantly increased the free PSA and reduced the total PSA of the test B groups. However, there was no significant decrease in prostatic ACP of the test animals in this group when compared to baseline as against comparison with the control group. Additionally, lower mortality rate of 33.3% was recorded. Other side effects such as loss of appetite and weight loss were minimized in this group when compared to test A group. These results are in agreement with the findings of Mohamad et al. (2015) and Torres et al. (2012), who independently showed that the n-hexane leaf extract of A. muricata negatively affected the viability of human pancreatic cancer cells.Furthermore, this investigation showed that the glycolipid fraction of A. muricata can be said to have no therapeutic effects whatsoever against indices of prostate cancer as it rather decreased the Free PSA and increased the total PSA of the test C group. There was equally no statistically significant decrease in the prostatic ACP in comparison with the control group and baseline groups. Mortality rate in the test animals in this group was very minimal, there was no significant side effect or potency of the fraction observed or recorded in the experimental animals. This result suggests that the glycolipid fraction of A. muricataextract lacks efficacy with respect to management of prostate cancer though it may be indicated for the management of other conditions.
5. Conclusions
- The simple lipid fraction of A. muricata has shown significant effect against prostate cancer indices in male healthy albino rats. Although the desired potency of the fraction may be said to be concentration dependent due to the toxicity and high mortality recorded, the result however suggests that a lower concentration of simple lipid fraction can be used as a pharmacological preparation for the management of prostate cancer. Similarly, the complex lipid fraction can also be used for the management of prostate cancer at its early stage as it showed high potent effects and reduced side effects. Due to the reduction in mortality and general toxicity accompanied by high potency recorded with the complex lipid fraction, it may be a better fraction of A. muricata leaf lipid for the management of prostate cancer. Finally, these observations further support the claim that A. muricata is effective against prostate cancer.
6. Recommendations
- Further purification and/or fractionation coupled with appropriate concentration adjustment of the complex and simple lipids are vital to the identification of the exact molecules exercising potency against prostate cancer and reduction of toxicity. The combination of the active components of the simple and complex lipid would clarify the observed activity and open up to possible synergistic reactions. There is need for this investigation to be carried out on cancer cell lines or on animals with disease state.
Ethical Approval
- The animals were handled following standard ethical guidelines on animal handling and research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML