-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2019; 7(1): 1-6
doi:10.5923/j.rct.20190701.01

Extracorporeal Immunotherapy Methods in the Treatment of Malignant Neoplasms
Kamishov S. V., Tillyashaykhov M. N.
Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Kamishov S. V., Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

For many years, cancer treatment has focused on surgery, chemotherapy, and radiation therapy, but as knowledge of the immune system's capabilities in the fight against cancer deepened, treatment methods using the immune system against malignant tumors began to develop. Although immunotherapy of malignant tumors is a relatively new scientific trend, the results obtained in the last decade show the important role of immunotherapeutic approaches in the treatment of oncological diseases. One of the methods that have been successfully used for stopping intoxication for a long time is therapeutic plasmapheresis, which consists of removing blood plasma containing antibodies, circulating immune complexes, cytokines, products of cellular metabolism, etc. Modern methods of extracorporeal immunopharmacotherapy in their nature are an effective extension of therapeutic plasmapheresis. If during plasmapheresis, cellular elements immediately after their separation from plasma are returned to the patient, then with extracorporeal immunopharmacotherapy, an additional release of the leukocyte fraction occurs, which is then subjected to treatment outside the body with a specific drug. Leukocytes activated in this way, after returning to the bloodstream, are able to synthesize the factors that activate the immune system and, consequently, activate other cells of the immune system. Thus, extracorporeal immunopharmacotherapy is a separate direction, born at the junction of immunology and extracorporal hemocorrection.
Keywords: Oncological diseases, Extracorporeal immunopharmacotherapy, Plasmapheresis
Cite this paper: Kamishov S. V., Tillyashaykhov M. N., Extracorporeal Immunotherapy Methods in the Treatment of Malignant Neoplasms, Research In Cancer and Tumor, Vol. 7 No. 1, 2019, pp. 1-6. doi: 10.5923/j.rct.20190701.01.
1. Introduction
- Historically, cancer immunotherapy began in 1891 when Dr. W. Coley for the first time at the Memorial Sloan-Kettering Cancer Center (USA, New York) tried to treat cancer patients with extracts from streptococcal cultures [1-2]. These attempts to use immunotherapy, of course, had a different outcome. Thus, in some patients the growth of tumors choked, but patients died due to the presence of complications from organs and systems affected by tumor intoxication, cachexia. But these attempts, which had some success, nevertheless predetermined a possible assumption about the damaging effect on the tumor of some factors unknown at that time that appear in response to the introduction of bacterial extracts.The research on antitumoral immunology (oncoimmunology) appeared much later, in 1957, when Prehn and Main first induced sarcoma in mice with methyl cholanthren, then began to study the role of the immune system in recognizing tumor antigens, controlling reproduction, differentiation and metastasis of tumor cells and their removal (elimination) from the body.In 1962, O'Malley and co-authors in experiments on mice proved that hemorrhagic necrosis in tumors after the introduction of bacterial lipopolysaccharide (LPS) is caused not by the action of LPS itself, but by some kind of intermediate factor that appears in the blood serum in response to LPS injection [3-4]. That blood serum had the ability to kill tumor cells when introduced to other mice that did not receive LPS injections. And finally, in 1975, again in the Memorial Sloan-Kettering Cancer Institute, E. Carswell and co-authors discovered and described a mediator with a cytotoxic effect on various tumor cells, appearing in the blood of mice in response to the introduction of LPS from Bacillus Calmette-Guerin and called it "Tumor necrosis factor" [1].During the 10 years of work on this program, the scientists of the Institute have developed a 3-step method for evaluating the effectiveness of cancer biotherapy methods. On the basis of this technique, the effectiveness of cancer biotherapy methods currently available (interferon alpha, beta, gamma, IL-1, IL-2, tumor necrosis factor (TNF), colony-stimulating factor (CSF), lymphokine-activated killer cells (LAC), tumor filtering lymphocytes (THL), monoclonal antibodies, growth factors) was studied in detail and the main directions for the creation of new methods of treatment of oncological diseases were identified [2]. Currently, immunotherapy is one of the most promising areas in the treatment of malignant tumors. It includes the treatment of tumors with monoclonal antibodies, anti-tumor vaccines, cytokines, activated lymphocytes, etc.Clear evidence and deciphering the mechanism of oncogenesis, knowledge of this process laws and the “control system” at the molecular level of regulation suggests that the pathogenetic process is affected not only from the point of application of the suppression of neoplasia cell growth, but from the position of the body’s immune response to the tumor process.So, historically, step by step, a new field of oncology – oncoimmunology appeared, the formation of which began with the discovery of oncogenes and the development of methods for the formation of a specific immune response against them [5-6].Despite the current and rapid development of oncoimmunology, today fundamental issues remain unresolved regarding the expression of specific antigens by human tumors, the immune response to this antigen, and the ability to influence this response with the aim of antitumor control and immune surveillance of the tumor process.The development of the oncological process in the body makes it possible to suspect the presence of a defect in the implementation of immune surveillance function allowing malignized cells to multiply unhindered. Secondly, the tumor cells themselves have a local and immunosuppressive effect due to the production of various substances. Thirdly, modern complex antitumor treatment (surgical, radiation and chemotherapy) have a powerful effect on factors that induce generalized immunosuppression. In this regard, a special group of patients who have been indicated to undergo immunotherapy with the aim of modification and correction are cancer patients. Usually, most patients have comorbidities, advanced age, immunosuppression which is further aggravated by radiation and / or several chemotherapy courses.The main theoretical preconditions for immunotherapy are the following: 1) tumor cells express antigens on the surface membrane that are different from normal; 2) in experimental carcinogenesis and in precancerous human diseases the immune system is deficient; 3) clinically detectable growth of neoplasms occurs in violation of the immune system state which is aggravated by antitumor treatment; 4) the higher reactivity of the immune system before and after treatment correlates with a better prognosis.The most difficult issue in the study of immunomodulators efficiency of is the correct assessment of the feasibility of introducing immunocorrective treatment and its effectiveness.It is clear that a progressive tumor causes serious changes in the immune response which the immune system alone cannot cope with and the inclusion of immunomodulators in the treatment of cancer patients is justified. In modern oncology the role of immunology has significantly expanded which provides new methods for diagnosing, monitoring and treating cancer as well as correcting the complications of traditional treatment.The basis of the new cancer treatment strategy is the principle of “complementary oncotherapy” [7], which implies a mutually reinforcing therapeutic effect of existing traditional and immunological therapies, taking into account the etiopathogenetic significance of each method. Immunological methods of treatment, in this case, are present at all stages of the antitumor treatment, but at each stage they perform various tasks. There is a nonspecific and a specific adoptive immunotherapy in modern oncology. The first one is designed to increase the capacity of immunocompetent cells due to transfused donor cells, the second one is to destroy the tumor in the recipient’s body by transfused lymphocytes with specific antitumor determinants. Unfortunately, clinical trials of these approaches are still sporadic and their implementation is still difficult due to technical problems, the toxicity of the complexes used, their unsufficient efficiency and other problems that have not been fully resolved yet.Active specific immunotherapy is an attempt to stimulate the immune response to antigens of a given tumor. This method uses vaccines created from tumor cells or tumor antigens.Antineoplastic vaccines may include whole tumor cells or only their antigens. To enhance the immunogenicity of the neoplasm cells are treated with substances acting on their membrane (neuraminidase, blockers of sulfhydryl groups, lipoidal substances, viruses, etc.) as well as using methods of hybridization of tumor and normal cells.In spite of encouraging results from earlier studies, randomized investigations of phase III have not confirmed the efficacy of oncolysate-based vaccines [9]. Numerous attempts of using sera for the treatment of various forms of tumors until very recently did not give unambiguous results. In some cases, they had a cytotoxic effect on the tumor while in others they led to an increase of tumor growth.Passive specific immunotherapy of malignant neoplasms includes methods and drugs having a highly specific effect on specific tumor antigens that carry tumor cells or tissues. One of these methods is to obtain monoclonal antibodies to tumor antigens and their use in conjugation with toxins or radioactive drugs. These drugs are called immunotoxins. Sometimes growth factors are used as carriers for toxins, and they are called oncotoxins.Recently, there have been many reports that are the subject of scientific debate about the of immunotherapy efficiency which is based on the use of monoclonal antibodies [10]. Monoclonal antibodies Herseptin (Trastuzumab) - recombinant monoclonal antibodies that selectively bind to receptors for human epidermal growth factor, HER 2. Gerseptin is recommended by the FDA as a first-line drug in combination with paclitaxel to treat some cases of metastatic breast cancer (with overexpression of the HER 2 protein) in patients who have not been prescribed chemotherapy. In cases where patients were prescribed chemotherapy drugs, Herseptin can be used as a monotherapy.Rituxan or Rituximab is a genetically engineered monoclonal antibody the target of which is the CD20 receptor located on some B-lymphocytes. B-lymphocytes marked with rituxan are destroyed by components of the immunity system, after which new B-lymphocytes are formed from stem cells. The process is terminated only after the complete elimination of all B-lymphocytes - carriers of the CD20 receptor.Monoclonal antibody to VEGF - Bevacizumab binds vascular endothelial growth factor (VEGF) and prevents its interaction with a specific receptor (Flt-1 and KDR) on the surface of endothelial cells. The use of bevacizumab as monotherapy or in combination with chemotherapy reduces tumor angiogenesis and suppresses the growth of solid tumors. Bevacizumab was approved by the FDA in February 2004 for the treatment of metastatic colorectal carcinoma as a first-line drug in combination with intravenous introduction of 5-fluorouracil.The use of extracorporeally cultured dendritic cells and fibrocytes as natural vectors for specific vaccination of cancer patients is at the core of another immunotherapy for cancer diseases. Currently, non-specific active immunotherapy is more widely used in the clinic and more developed than other types of immunotherapy. Bacterial vaccines, polysaccharide preparations (Zymosan, Manosin, Permyl, Glucan, Prodigiosan, Pyrogenal), interferon and thymus biologically active factors, inducers of endogenous interferon formation and serum serum activity, etc., are used as non-specific stimulants.The first report on the clinical use of IL-2 in patients with melanoma was in 1983. These early studies were based on known immunological effects obtained in experimental conditions.Systemic introduction of IL-2 led to the proliferation of lymphocytes and an increase in the cytotoxic antitumor activity of T-killers. In clinical studies IL-2 was used alone or in combination with LAK cells in patients with disseminated forms of cancer.The first clinical effects were found with the introduction of IL-2 in melanoma patients. In 1987 S. Rosenberg in the treatment of 46 melanoma patients with high doses of IL-2 reported 16 cases of objective effect. High-dose IL-2 immunotherapy was accompanied by evideent side effects. The earliest of them were fever and chills. In a number of patients, IL-2 therapy caused gastritis which required the introduction of H-2 receptor blockers into the treatment regimen. High doses of IL-2 led to fluid retention in the body caused by oliguria, hypotension and impaired vascular permeability. Hemodynamic disturbances and other complications were also observed. Anemia, leukopenia and thrombocytopenia were observed at the indicated doses of IL-2 in 57-81%.Research of S. Rosenberg, conducted in 1986-1987 in the surgical department of the National Cancer Institute in Philadelphia (USA) using interleukin-2 and the additional introduction of lymphokine-activated killer cells (LAC) showed the possibility of achieving complete regression of renal cell cancer metastases in 33% of patients, which in turn was reflected in survival rates.The main role in antitumor protection is played by a certain group of lymphocytes called natural killers (NK). However, their number is not large, only 10-15% of all blood lymphocytes which does not allow NK to cope with the tumor mass.The proposed methods allow to obtain from a normal lymphocyte of the patient’s blood a significant amount of LAK cells which are introduced into the patient’s body, where they perform an antitumor effect. LAC cells in the total volume of peripheral blood are in a small amount and have relatively low specificity for the tumor in which they are used. Therefore, this technique can be attributed to a greater extent to non-specific immunotherapy.In subsequent studies the total tumor regression in patients with kidney cancer, melanoma and colorectal cancer after IL-2 / LAC therapy was 35%, 21% and 17%, respectively. At the same time, there are cases when the duration of remission exceeded 5 years.In 1990 a randomized study conducted by the NCI (181 cases) showed that the combination of IL-2 with LAC was more effective than the IL-2 therapy [11]. In particular, complete regressions in IL-2 / LAC therapy made up 10%, while the introduction of high doses of IL-2 caused this effect in 3% of patients, and the actual three-year survival of patients was 37% and 21%, respectively.Clinical studies of the use of recombinant g-interferon have shown that the drug enhances the antitumor effect of cytotoxic lymphocytes and together with lymphotoxin suppresses the growth of tumor cells. By acting on the nucleus of the tumor target-cell, g-interferon induces the expression of lymphotoxin receptors on it. In addition, g-interferon inhibits angiogenesis which deprives the tumor of the flow of nutrients and thereby slows down its growth.T-activin and its analogue Timalin is widely used in oncology, including oncogynecology. These drugs are a complex of polypeptide fractions extracted from the thymus gland of cattle. The drugs are used as an immuno-and biostimulator in the complex therapy of diseases accompanied by a decrease in cellular immunity, as well as in suppression of immunity and hematopoietic function after radiation or chemotherapy in cancer patients. The use of T-activin in patients with ovarian cancer (OC) reduces the number of postoperative complications, the frequency of mielodepressive conditions in the background of the use of cytostatics and improves the clinical course of the disease.Thymogen - a dipeptide, also obtained from the thymus gland of cattle, has a predominant effect on the T-system of immunity. It is indicated for the prevention of radiation therapy and chemotherapy complications, accompanied by inhibition of cellular immunity. Side effects and contraindications are not currently identified.The team of researchers from the Research Institute for Experimental Diagnosis and Therapy of Tumors (Russian Academy of Sciences) in 1992 developed a method of adaptive photoimmunotherapy based on extracorporeal irradiation of leukocyte mass extracted from the patient's blood. It uses a helium-neon laser (wavelength - 632.8 nm). Subsequently, the leukocyte mass is reinfused into the bloodstream. Then T-lymphocytes are stimulated. This method was applied in patients with breast cancer, in whom the time of metastasis in the postoperative period was slowed down. It was found that the immunomodulatory effect of the laser depends on the patient's condition: the more evident suppression of the immune status causes the more effective treatment. At the same time, prolonged and uncontrolled blood irradiation leads to inhibition of immunity and the development of an immunosuppressive state.The main mechanisms of the therapeutic effect of plasmapheresis as an extracorporeal method of immunotherapy include: 1) a decrease in the concentration of toxins; 2) hemodilution, improved circulation; 3) reduction of the content in the circulation of significant antigens and antibodies to them; 4) reduction of the level of immunoglobulins, circulating immune complexes, acute phase proteins, etc.; 5) transfer of circulating immune complexes, antigens and antibodies from the tissue to the circulating pool; 6) immune cell receptor de-blockade; 7) improving the effectiveness of agents that affect the immune system. The plasmapheresis procedure is accompanied by hemodilution, as evidenced by a decrease in hematocrit by 15% and total protein concentration by 20%. At the same time, compared with the initial data, blood viscosity decreases by 19%, fluidity increases by 20%, electrophoretic mobility of erythrocytes - by 22%, their aggregation ability decreases by 35%, platelets - by 32%. Plasmapheresis eliminates the blockade of the macrophage system and simultaneously optimizes the functions of damaged organs. The receptor sensitivity to hormones is restored (both its own endocrine system and introduced into the body), receptors that bind to drugs are unblocked, which explains the increased sensitivity of the body to drug therapy.One of the mechanisms that provide the therapeutic effect of plasmapheresis is the deplassing of cellular elements. Along with the plasma, pathological elements adsorbed on the cell surface are removed, the vital activity of the cells changes, new interactions with other cells and regulatory facts arise.There is a dynamic equilibrium of concentrations of substances in the intracellular, extracellular and intravascular spaces. A change in concentration in one of them (in this case, in the intravascular space) leads to a redistribution in the others. Therefore, immediately after plasmapheresis, a significant decrease in the level of pathological products is observed, but after a few hours their content increases due to the intake of substances from the vascular bed that were previously in the interstitium or even in cells. The following plasmapheresis sessions contribute to the removal of these metabolites and lead to an evident therapeutic effect, since the main part of the harmful products is in extravascular spaces.Modern methods of extracorporeal immunopharmacotherapy (EIPHT) are inherently an effective extension of therapeutic plasmapheresis. EIPHT is carried out by the exfusion of 200-250 ml of auto blood into “Gemakon” or “Terumo” sterile containers, incubation with immunomodulators: thymalin in a total dose of 60 mg (3 procedures), cycloferon in a total dose of 750 mg (3 procedures) or polyoxidonium in a total dose of 36 mg (3 procedures) at 37°C for 60-100 minutes followed by reinfusing of the resulting conjugate (Fig. 1).
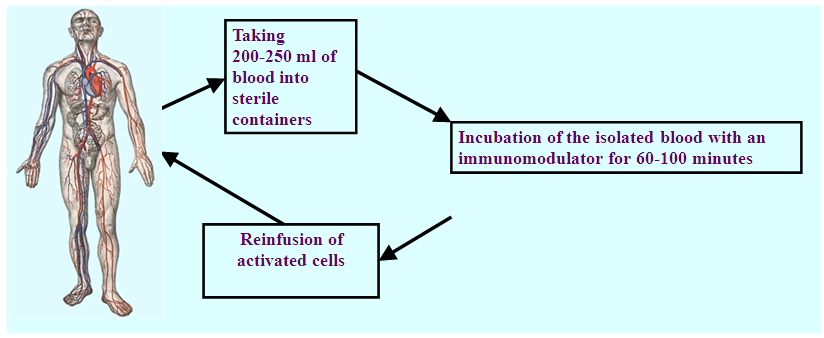 | Figure 1. EIPHT technique without plasma exchange |
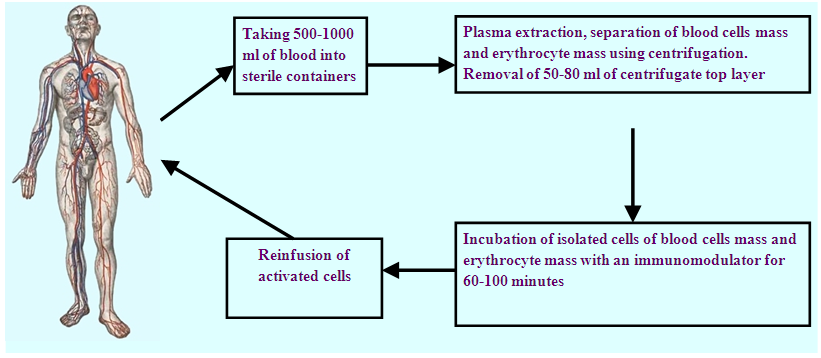 | Figure 2. EIPHT technique with intermittent plasmapheresis |
2. Summary
- However, there is very little information in the literature about the use of the EIPHT method in the treatment of cancer. An analysis of current literature data makes it possible to conclude [12-13] that the immune system is at the center of all attempts currently being made to improve the effectiveness of antitumor therapy and the task of activating the antitumor potential of the immune system is the most important in modern oncology. The application of modern unique immunomodulatory drugs has created qualitative new base for the correction of immunity disorders. Numerous studies have shown that modern methods of immunotherapy in the treatment of malignant tumors can have a normalizing effect on the immune status of cancer patients and provide an objective antitumor effect.
3. Conclusions
- Blood includes all the essential elements of both innate and acquired immunity. Blood leukocytes (granulocytes, monocyte / macrophages and lymphocytes) are responsible for cellular immunity. The effect on leukocytes by immunomodulating drugs under ex vivo (outside the body) conditions can dramatically increase their migration and functional activity. Extracorporeal use of the drugs allows to impact purposefully on immunocompetent cells and to minimize their effect on internal organs and tissues of the body. Extracorporeal immunotherapy can be effectively used for the treatment of timorous, infectious, autoimmune and degenerative diseases including the cases when the possibilities of standard treatment methods are exhausted.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML