-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2018; 6(1): 5-12
doi:10.5923/j.rct.20180601.02

Role of Galactose Specific Lectin 3 and CYP17A1 Gene Polymorphism in Breast Cancer
Eman M. I. Youssef1, 2, Nisreen Aref Albezrah3, Amgad A. Ezzat4, Wael F. Sedik5, Emad E. Radwan6, Aml El-Sayed Abdou Yehia7, Rehab M. Elsaid Tash8, Mohamed I. Ahmed9, Alaaeldeen M. Elbahaie10
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Medical Biochemistry, College of Medicine, Taif University, KSA
3Head of Department of Obstetrics and Gynecology, College of Medicine, Taif University, KSA
4Department of Microbiology & Immunology, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
5Department of Medical Biochemistry, Faculty of Medicine, Minia University, Egypt
6Department of Obstetrics and Gynecology, Suez Canal University Hospitals, Egypt
7Department of Microbiology & Immunology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
8Department of Microbiology & Immunology, Zagazig Faculty of Medicine, Zagazig, Egypt
9Department of Obstetrics and Gynecology, Benha Faculty of Medicine, Benha University, Benha, Egypt
10Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Suez Canal University, Egypt
Correspondence to: Eman M. I. Youssef, Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Breast cancer (BC) is the most frequent malignant tumor and is considered the second leading cause of cancer related deaths among women worldwide. Galactose specific lectin 3 (Gal-3) is a β-galactoside-binding animal lectin that contains carbohydrate-recognition domains and displays multiple related functions. The human CYP17A1 gene, which encodes the enzyme cytochrome P450 17A1, plays a key role in sex steroid synthesis. Objective: The aim of this study was carried out to evaluate the role of serum Gal-3 as a diagnostic tumor marker in BC and to assess the potential association between the CYP17A1 gene polymorphism and BC. Subjects and Methods: The present study was conducted on 35 breast cancer females patients compared to a control group of 35 healthy females of matching ages. Estimation of Gal-3 in serum using enzyme linked immune-sorbent assay (ELISA) technique and detection of CYP17A1 gene polymorphism using polymerase chain reaction - restriction fragment length polymorphism technique (PCR – RFLP) were performed in all participants. Result and Conclusion: The results of this study suggest that the Gal-3 showed increase in breast cancer cases and could be used as a useful marker of BC. On the other hand there was no significant association between CYP17A1 gene polymorphism and BC.
Keywords: Breast cancer (BC), Galactose specific lectin 3 (Gal-3), Cytochrome P450 17A1(CYP17A1)
Cite this paper: Eman M. I. Youssef, Nisreen Aref Albezrah, Amgad A. Ezzat, Wael F. Sedik, Emad E. Radwan, Aml El-Sayed Abdou Yehia, Rehab M. Elsaid Tash, Mohamed I. Ahmed, Alaaeldeen M. Elbahaie, Role of Galactose Specific Lectin 3 and CYP17A1 Gene Polymorphism in Breast Cancer, Research In Cancer and Tumor, Vol. 6 No. 1, 2018, pp. 5-12. doi: 10.5923/j.rct.20180601.02.
Article Outline
1. Introduction
- Globally breast cancer (BC) accounts for more than 400.000 deaths per year [1]. However, despite considerable diagnostic and therapeutic advances in recent years, it is still the most common female cancer in the United States, and the main cause of death in women ages 40 to 59 years [2]. Approximately 40% of patients with localized breast cancer are difficult to detect at the time of diagnosis and treatment especially after surgery and leading to disease recurrence and death several years after diagnosis [3]. Convenient tumor markers may be able to define risks and identify the early stages of tumor development, assist in tumor detection and diagnosis, monitoring of disease, as well as the treatment response prior to development of clinically notable signs and developing novel therapies. In addition to diagnosis, the biomarker can predict outcomes of the disease, help in surveillance for disease recurrence and achieve a greater specificity and sensitivity in the follow up of malignancy [4]. Galactose specific lectin 3 (Gal-3), also called galectin-3 is approximately a 30 kDa -galactoside-binding protein belonging to the Galectin family [5], which consists of more than ten members. Gal-3 is composed of a carboxyl-terminal carbohydrate-recognition-binding domain (CRD) and amino-terminal tandem repeats of about 130 amino acids. Gal-3 is encoded by a single gene, LGALS3, located on chromosome 14, locus q21–q22 and normally is expressed in the nucleus, cytoplasm, mitochondrion, cell surface, extracellular space [6, 7] and is distributed in epithelia of many organs such as activated macrophages, eosinophils, neutrophils, mast cells, epithelium of the gastrointestinal and respiratory tracts, kidneys and some sensory neurons, and is also involved in tumorigenesis, angiogenesis, and tumor metastasis [8, 9].The expression of this human lectin displays multiple important biological functions such as embryogenesis, growth, cell adhesion, proliferation, differentiation, cell-cycle progression, apoptosis, mRNA splicing, and regulation of the immune system [10, 11]. Secreted Gal-3 plays an important role in the progression of breast cancer and is significantly elevated in the sera of breast cancer patients compared to healthy controls [12, 13]. BC is a multifactorial disease related to genetic, environmental and life style factors that influence the level of exposure to estrogen which may play a central role in development of breast cancer [14, 15]. The association between exposure to steroid hormones especially estrogen and breast cancer is well established. Estrogen biosynthesis, cellular binding and metabolism involve many steps, and the genes controlling these steps may contribute to inherent variability in breast cancer susceptibility [16].Genetic polymorphisms in genes involved with hormone metabolizing pathways have been widely studied for evidence of their contribution to breast cancer risk [17, 18]. One such gene is CYP17A1 on chromosome 10, which codes for the human enzyme cytochrome P450 17A1, also called steroid 17α-monooxygenase or 17α-hydroxylase and plays a key role in sex steroid synthesis, mainly related to estrogen biosynthesis pathway [19]. CYP17A1 is a 57.4 kDa protein that composed of 508 amino acid residues. Cytochrome P450 17A1 is expressed in many tissues and cell types, including the zona-reticularis of the adrenal cortex and zona-fasciculata as well as gonadal tissues [20, 21].One of its most commonly studied polymorphism is the T→C nucleotide substitution 34bp upstream of the translation site in the promoter region, which creates a recognition site for MspA1 restriction enzymes [22, 23]. This SNP was originally thought to create an additional Sp-1 binding site in the 5' promoter region of the gene which could lead to transcriptional activation and higher activity of the enzyme which may influence susceptibility to breast cancer [24]. The objectives of the current study are to verify the possibility of using serum galactose specific lectin 3 (Gal-3) level as a potential diagnostic tumor biomarker for breast cancer and to assess the potential association between the human CYP17A1 gene polymorphism and breast cancer.
2. Subjects and Methods
2.1. Subjects
- The study included seventy subjects; their ages ranged 27 - 75 years. Patients were attending the outpatient clinics of Obstetrics and Gynecology Departments of Suez Canal and Benha Universities Hospitals. They were classified into two groups as follows: Group I: included thirty five newly diagnosed female patients with breast cancer (BC), not subjected to surgery, chemotherapy or radiotherapy. They were diagnosed by clinical examination, mammographic criteria and histo-pathological typing. Their ages ranged between 33-75 years (mean age 55±20 years). In addition thirty five apparently healthy females as a control group and their ages ranged between 27-66 years (mean age 45.8±19.9 years). This study was performed after obtaining informed consent from all participating subjects enrolled in the study.All participants were subjected to the following: Full history taking including: age, age at diagnosis (in case of patients only), family history of breast cancer, age at menarche and/or menopause, parity, number of pregnancies, history of breast feeding and use of oral contraceptive pills (OCP), complete clinical examination, diagnosis of breast cancer (in case of patients only) through mammographic grading and histological staging of the tumor according to American Joint Committee on Cancer [25], assay of serum galactose specific lectin 3 (Gal-3) quantitatively using sandwich enzyme linked immunosorbent assay (ELISA) technique using ELISA Kit (Catalog number: BMS279/4) supplied from eBioscience International, Inc., Europe and detection of CYP17A1 gene polymorphism using polymerase chain reaction – Restriction fragment length polymorphism technique (PCR – RFLP).
2.2. Methods
- Specimen Collection:Five millilitres venous blood samples were withdrawn from each subject under complete aseptic precautions in plain vaccutainer tubes and divided into 2 aliquots: Three millilitres were withdrawn into sterile EDTA vacutainer tubes, properly mixed then stored at -20ºC for DNA extraction. The second one: Two ml, left to clot, centrifuged at 1000xg for 10 minutes and then the sera were separated for the galactose specific lectin 3 (Gal-3) which was stored at -20° C till the time of assay.
2.3. Detection of Human Serum Galactose Specific Lectin 3 (Gal-3) Assay by ELISA Method
- Human Gal-3 present in the sample or standard binds to antibodies adsorbed to the microwells. A biotin-conjugated anti-human Gal-3 antibody is added and binds to human Gal-3 captured by the first antibody. Following incubation unbound biotin-conjugated anti-human Gal-3 antibody is removed during a wash step. Streptavidin-HRP is added and binds to the biotin-conjugated anti-human Gal-3 antibody. Following incubation unbound Streptavidin-HRP is removed during a wash step, and substrate solution reactive with HRP is added to the wells. A colored product is formed in proportion to the amount of human Gal-3 present in the sample or standard. The reaction is terminated by addition of acid and absorbance is measured at 450 nm. A standard curve is prepared from 7 human Gal-3 standard dilutions and human Gal-3 sample concentration determined.
2.4. Genomic DNA Analysis of CYP 17 – Msp A1I Polymorphisms using Polymerase Chain Reaction – Restriction Fragment Length Polymorphism (PCR – RFLP)
- The test was done in five main steps: A- Extraction of genomic DNA from peripheral blood leucocytes of EDTA anti-coagulated blood. This was performed using DNA extraction kit QIAamp (DNA Blood Mini Kit) supplied by QIAGEN: 181 Bay Street, Suite 4400, Toronto, Ontario, H4J2T3. B- DNA Amplification Using the Polymerase Chain Reaction (PCR): Amplification of the extracted DNA for CYP17A1 [26]. PCR Master Mix (supplied by Fermentas Life Sciences) is an optimized ready-to-use PCR mixture of Taq DNA Polymerase, PCR buffer, MgCl2 and dNTPs. PRIMERS (supplied by Bioneer): Two primers were used to detect the MspA1I polymorphism in the CYP17A1 gene. According to the manufacturer’s instructions, the primers were prepared so that each 1 μL contains 20 pmole of the primer. After their preparation, they were stored at -20°C. Forward (F) and reverse (R) sequences were as follows: 5'– CAT TCG CAC TCT GGA GTC – 3' (for the forward primer) and 5' – AGG CTC TTG GGG TAC TTG – 3' (for the reverse one). Enzymatic amplification was performed by PCR using Taq polymerase enzyme in a Hybaid thermal cycler (Promega Corporation, USA). The computerized thermocycler was programmed for the following thermal conditions which were proposed by [26] for CYP17A1-MspA1I detection: i- Initial denaturation at 94ºC for 5 minutes. ii- 35 cycles of amplification consisting of: Denaturation at 94ºC for 1 minute, annealing at 57ºC for 1 minute and extension at 72ºC for 1 minute. iii- A final extension step at 72ºC for 10 minutes. C- Detection of PCR amplification products using agarose gel electrophoresis containing ethidium bromide and ultraviolet light transillumination: The amplified samples were run in parallel on 1.5% agarose gel stained with ethidium bromide using gel electrophoresis and visualized on a UV transilluminator to detect the presence of amplified product. D- The amplified products were digested with the suitable restriction enzyme specific for the polymorphism (Msp A1 I): After PCR reaction analysis was performed, the amplified product was digested with the specific restriction enzyme [Msp A1I restriction enzyme for T→C (-34 bp) CYP17 promoter] and the digested products were separated by 3% agarose gel electrophoresis and visualized by UV transilluminatation. According to the manufacturer’s instructions protocol was performed for digestion of PCR products after amplification. E- The digested products were then analyzed by electrophoresis on agarose gel containing ethidium bromide, and visualized by ultraviolet transilluminator for identification of the polymorphism in the CYP17A1 gene. Detection of the products of digestion of CYP17A1 – MspA1 I polymorphism in agarose gel using ultraviolet transillumination: The substitution of C for T at -34 bp of gene CYP17A1 creates a new restriction site for the endonuclease MspA1I, which cleaves the 459 bp. PCR product into two fragments of 335 bp and 124 bp. The subject was considered to carry: The wild type gene (TT) if only one band appears at 459bp. Homozygous polymorphism (CC) if two bands appear at 335bp and 124bp. Heterozygous polymorphism (CT) if the 3 bands appear at 459bp, 335bp and 124bp.
2.5. Statistical Methods
- The results were analyzed using the SPSS computer software package, version 17 (SPSS Inc., Chicago, IL). Qualitative data were presented in the form of frequencies and percentiles whereas quantitative data were shown as mean and standard deviation or median and range as appropriate. The Receiver Operating Characteristic (ROC) curve was used for prediction of cut off values. A p-value < 0.05 was considered significant difference; < 0.01 was considered highly significant difference, > 0.05 means non-significant difference.
3. Results
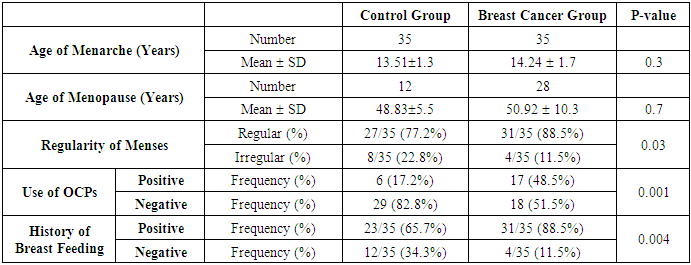
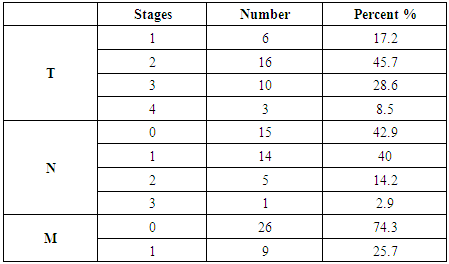
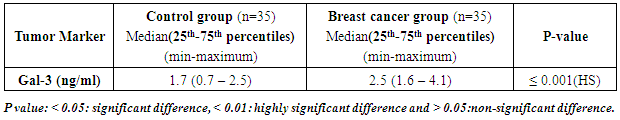
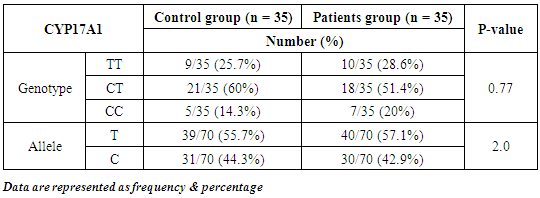
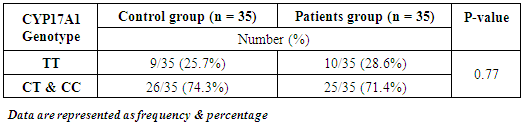
- Results of the present study are shown in tables (1-5) and figures (1-3).Table (1) shows group characteristics in the studied groups. The age of female cancer breast patients at the time of diagnosis was 45.6 ± 7.9 years. Only 7/35 (20%) had positive family history. Table (2) shows the descriptive characteristics of tumor stage (T), lymph node metastasis (N) and distant metastasis (M) in breast cancer group. Figure (1) shows frequency of pathological grades of breast cancer group. In addition, statistical comparison between the galactose specific lectin 3 (Gal-3) tumor marker in breast cancer patients and the healthy control group revealed a highly significant increase in Gal-3(P <0.001) (Table 3). Receiver operating characteristic (ROC) curve analyses was applied to assess the diagnostic utility of Gal-3 in breast cancer patients versus healthy control group. The best cutoff level of Gal-3 was 2.44 ng/ml, as diagnostic sensitivity was 85%, diagnostic specificity was 79.5%, negative predictive value was 81% and positive predictive value was 76% (Figure 2). Regarding the prevalence of the different genotypes, the results of this study found that the TT genotype was prevalent in 9/35 (25.7%) of the controls and 10/35 (28.6%) of the patients. The CC genotype was prevalent in 5/35 (14.3%) of the controls and 7/35 (20%) of the patients while the CT genotype was prevalent in 21/35 (60%) of the controls and 18/35 (51.4%) of the patients. No association could be detected on comparing the proportions of the two alleles (T, C) among the controls and the patients (p= 2.0 as shown in Table 4). On comparing the two studied groups regarding the distribution of CYP17A1 genotypes; wild type (TT) versus polymorphic genotypes (CC; homozygous and CT; heterozygous polymorphisms), no statistically significant association was found between the presence of polymorphic genotypes and the presence of cancer breast (p = 0.77 as shown in Table 5).
|
|
|
|
|
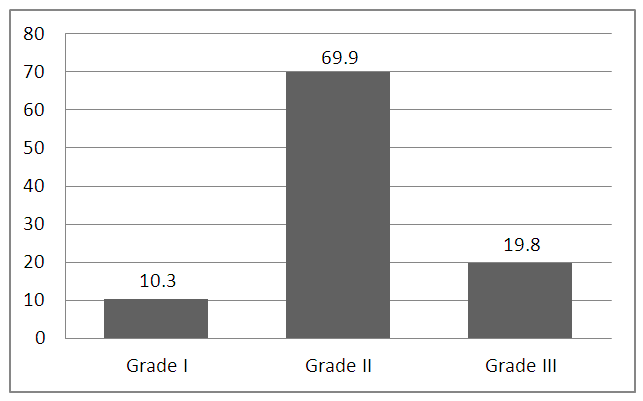 | Figure (1). Grade of the studied breast cancer cases |
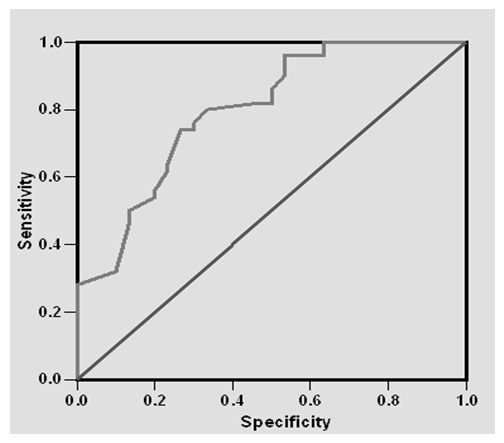 | Figure (2). Receiver Operating Characteristics (ROC) curve of Gal-3 for diagnosis of breast cancer |
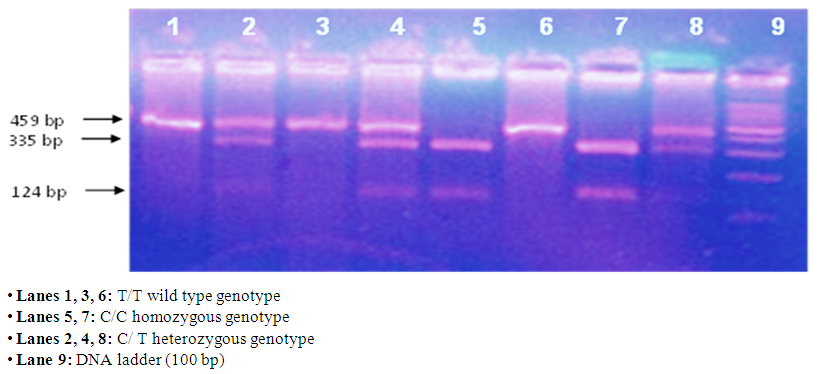 | Figure (3). Gel electrophoresis showing PCR-based RFLP analysis of the genotypes of CYP17A1- T>C gene polymorphism |
4. Discussion
- Breast cancer (BC) is one of the most common neoplasms and the second leading cause of cancer-related deaths among women worldwide [3]. The results of the present study showed that no statistical significant difference between the cases and the controls regarding the mean age of menarche (p=0.30) and the mean age of menopause (p=0.71). In agreement with our results, Jenny et al. [27] and Ghiasvand et al. [28] who showed no significant association between late age at menopause (>55years) and breast cancer. In addition, Chang et al. [29], Yueping et al. [30] and Jenny et al. [27] had not detected such associations shown in this study. On the opposite side, two studies conducted in 2013 had concluded that late menopause was significantly associated with breast cancer risk among Chinese women [31, 32]. However, some studies demonstrated a long lasting influence of early age at menarche on breast cancer [33, 34]. An early age at menarche translates into an earlier estrogen exposure and breast epithelial growth [35]. Regarding the menstrual regularity, this study showed a statistically significant difference between bothpatients and control groups (p=0.03). The initiation of regular ovulatory cycles is possibly the most critical reproductive determinant of breast cancer risk. Previous studies of [36] showed that women with early menarche and rapid establishment of regular menstrual cycles had an almost 4-fold increased risk of breast cancer when compared with women with later menarche and long duration of irregular cycles. Furthermore, delayed menopause maximizes the number of ovulatory cycles leading to increased risk of breast cancer by 3% for every one-year increase in age of menopause [37].In the current study, we found that serum level of galactose specific lectin 3 (Gal-3) was increased and highly statistically significant in breast cancer group compared to control group (P≤0.001). The present study results are in consistent with the findings reported by Zhao et al. [38] who demonstrated that Gal-3serum levels in patients with BC were significantly higher than those in healthy individuals (P=0.01) and showed that the increased circulation of Gal-3 in the bloodstream of breast cancer patients promotes several important steps of the metastatic cascade. Furthermore, a group of researchers, Chen and colleagues [12] studied the expression of Gal-3 in breast tissues in both malignant tumor and benign lesions as controls in 2010. According to their study results, the positive rates of Gal-3 were significantly higher in breast cancer tissue than those in benign lesions (controls). These results agreed with the current results and the study of Matarrese et al. [39], who investigated the expression of Gal-3 on breast cancer tissue using immune histochemical technique and revealed over-expression of Gal-3 in breast cancer than normal breast tissue.Other studies conducted about the role of Gal-3 in other malignancies showed that determination of Gal-3 levels in urine of ovarian cancer patients and healthy controls established a strong correlation between the stages of the disease with Gal-3 concentration [40]. Data obtained by Sakaki et al. [41] demonstrated that serum levels of Gal-3 in patients with bladder cancer are significantly higher than the control group. In the group of patients with melanoma, serum Gal-3 concentration was shown to be significantly higher compared with healthy volunteers [42]. Northern blotting and Western blotting analysis done by Berberat et al. [43] showed significantly higher Gal-3 mRNA and protein levels in pancreatic cancer samples compared to normal controls. In thyroid cancer, most investigations support usefulness of Gal-3 as a biomarker for differentiated thyroid cancers [44]. Although studies confirmed that serum Gal-3 is directly proportional to tumor stage and metastatic cascade, this current study failed to prove that which could be due to insufficient sample size.In addition, The MspA1 polymorphism of CYP17A1 was investigated in our study. The percentage of the three resulting genotypes (TT, CT and CC) was 25.7%, 60% and 14.3% in the control group while in BC group; it was 28.6%, 51.4% and 20% respectively. The prevalence of the T and C allele was 55.7% and 44.3% among the controls with 57.1% and 42.9% among BC patients respectively. The results of this study were similar to previously published frequencies [45, 46]. In a study conducted by Einarsdottir and his Colleagues [47], on Swedish women, the proportions of TT, TC and CC among controls were 36.5%, 47.7% and 15.8% respectively which is near to the present results. However, the prevalence of the wild genotype (TT) was lower in the current studied population. In our study no significant differences were detected in either the genotype distributions or the allele frequencies between the control subjects and breast cancer patients. The findings of the present study were in agreement with previous study conducted by Dunning et al. [48] who found no significant differences in either the allele frequencies or the genotype distributions between breast cancer cases and control subjects (p = 0.35; 0.65 respectively) in British population. Verla-Tebit et al. [49] reported that the prevalence of the variant C allele was approximately similar among the breast cancer cases and controls (43% and 41% respectively) in German study population. Feigelson et al. [50] and Einarsdottir et al. [47] detected no statistically significant difference in genotype frequency between breast cancer cases and controls (p=0.7 and 0.6 respectively).In contrast to the results of the present study, Surekhaet al. [51], found that the TT genotype frequency was increased in breast cancer patients (68.7%) when compared to controls (55.4%).The results of many studies of the association between MspA1 variant and breast cancer were inconsistent. This may be in part due to difference in study methods, some studies were not population based, which renders them susceptible to the effects of selection bias [52]. Also studies differ markedly in size and so they varied in statistical power [51]. In the view of the current data, the present study provides the evidence that the serum levels of the Gal-3 showed promising increase in breast cancer cases and could be used as a useful marker of BC.On the other hand, there was no significant association between CYP17A1 gene polymorphism and breast cancer. No association between the above mentioned patient characteristics and CYP17 genotypes was noticed. Further studies on the correlation between Gal-3 and tumor staging, employing tissue expression rather than serum levels are recommended in breast cancer patients. Further studies are also recommended to evaluate the effects of polymorphism in multiple genes involved with the sex hormone metabolic pathways on susceptibility to breast cancer. The use of larger sample size is recommended in further studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML