-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2018; 6(1): 1-4
doi:10.5923/j.rct.20180601.01

CALR Mutations Type 1 and Type 2 in Unmutant JAK2 Myeloproliferative Neoplasms in Sudanese Patients
Rania Hassan Mohamed1, 2, Ibrahim Khider Osman1, Enaam Abdel Rhman Abdel Gader3, 4, Adil Ahmed MD5, Awad Omer Ahmed6
1Department of Hematology, Faculty of Medical Laboratory Science, Alneelin University, Khartoum, Sudan
2Department of Hematology, Faculty of Medical Laboratory Science, University of Medical Science and Technology, Khartoum, Sudan
3Department of Pathology, Faculty of Medicine, Alneelin University, Khartoum, Sudan
4Radioisotope Center of Khartoum, Khartoum, Sudan
5Duke University Health Center, Durham, North Carolina, USA
6Royal Care International Hospital, Alneelin University, Khartoum, Sudan
Correspondence to: Rania Hassan Mohamed, Department of Hematology, Faculty of Medical Laboratory Science, Alneelin University, Khartoum, Sudan.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: During the previous 12 years, key improvement has been gifted with the encounter of activating mutations that are associated with the majority of BCR-ABL negative human myeloproliferative neoplasms (MPNs). In 2005 the discovery of JAK2 V617F generated great attention in the JAK2-STATpathway. Followed by detection of mutation in the thrombopoietin receptor (MPL) is a hallmark of MPNs. Later in 2013, mutations in the gene coding for the chaperone calreticulin were described in 20–30% of essential thrombocythemia and primary myelofibrosis patients. Consequently, genotyping for CALR mutation represents a novel, useful tool for an accurate diagnosis, prognosis and therapeutic relevance. In addition to that, CALR mutations are expected to be included in the diagnostic criteria for MPN (especially ET and PMF) in the upcoming WHO classification. Now, we will address the question: what do we know about calreticulin that could help us understand its role in MPNs? What is the frequency of this mutation among UN mutant JAK2 MPNs Sudanese patients? Material and methods: 53 male, 47 female was included in this study. A well-structured questionnaire was used to collect the data. DNA was extracted from blood samples using standard techniques, according to the manufacturer’s instructions. CALR mutations were analyzed using allele specific PCR. Data were analyzed by SPSS software (ver. 23). The results: Among the participants, the total JAK V617F negative patients were 37 (36.6%), The overall CALR mutations were detected in 15 patients (40.5%) in which CALR mutations were detected in 4/37 (10.8%) and 11/37 (29.7%) with CALR mutation type 2. Of the 37 patients tested. 8 of the patients (53.3%) of CLAR positive individuals, were diagnosed with ET, 5/15 (33.4%) diagnosed with MF and 2/15 (13.3%) diagnosed with PRV. Conclusions: CALR could be a worthwhile diagnostic tool for JAK2 -or MPL -negative ET or PMF patients. CALR mutation may be a diverse disease group, with different hematological characteristics than that of JAK2 -positive patients.
Keywords: Myeloproliferative neoplasms, CALR, JAK2, Sudan
Cite this paper: Rania Hassan Mohamed, Ibrahim Khider Osman, Enaam Abdel Rhman Abdel Gader, Adil Ahmed MD, Awad Omer Ahmed, CALR Mutations Type 1 and Type 2 in Unmutant JAK2 Myeloproliferative Neoplasms in Sudanese Patients, Research In Cancer and Tumor, Vol. 6 No. 1, 2018, pp. 1-4. doi: 10.5923/j.rct.20180601.01.
1. Introduction
- The human myeloproliferative neoplasms (MPNs) are a group of clonal stem-cell malignancies characterized by overproduction of mature blood cells. [1, 2]. According to the World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues, [3] the MPN subtypes: polycythemia Vera (PV) essential thrombocythemia (ET) and primary myelofibrosis (PMF) [4].The calreticulin (CALR) gene, found on chromosome 19p13.3, Functionally, CALR is believed to contribute in Ca2+ homeostasis as a calcium-binding protein, handling misfolded proteins, cell adhesion, immune response to cancer, and phagocytosis [5]. In 2013, somatic CALR mutations were identified in most JAK2 unmutated patients with essential thrombocythemia or primary myelofibrosis patients. Multiple CALR mutations that generated a +1 bp frame shift and resulted in mutant proteins with a novel C-terminus were verified in exon 9. One insertion (K385fs*47) and one deletion (L367fs*46) mutation were particularly common (together constituting 85% of CALR mutations) [6, 7] The role of CALR in the pathogenesis of MPN is largely unknown. Klampfl and colleagues and Mongolia and colleagues smartly established that CALR mutations are acquired, early in the major clone. [8]. Mutant CALR activates JAK/STAT signaling henceforth generating a proliferative signaling [8]. In addition, the forced expression of mutant CALR in hematopoietic cell lines resulted in MPL-dependent activation of MAPK signaling. Accordingly, patients with CALR mutations showed increased MAPK activity in blood cells and in CD34+ cells, leading to enhanced megakaryopoiesis and pro-platelet production. Recently it was also shown the IL3 independent growth of CALR mutant cells [9-11].CALR mutations have been described as mutually exclusive with JAK2 and MPL mutations and are present in a percentage of patients ranging from 56 to 88% of JAK2/MPL-negative cases [12]. The evaluation of CARL mutations increases the diagnostic accuracy in patients without other molecular markers and could represent a new therapeutic target for molecular drugs [13]. The aim of this study was to determine the prevalence, biological characteristics, and clinical correlations of these novel CALR mutations in patients with ET and PMF.DNA extraction:DNA purification kits (analytikjena, Germany) were used to isolate the DNA according to manufacturer’s instructions.
2. PCR
- Allele specific PCR was carried out to detect type 1 and type 2 CALR mutations using the following primers forward primer 1: 5´-GCA GCA GAG AAA CAA ATG AAG G-3´ for type 1 mutation, forward primer 2: 5´-GCA GAG GAC AAT TGT CGG A-3´ for type 2 mutation, and reverse primer: 5´-AGA GTG GAG GAG GGG AAC AA-3´ as a general primer. The PCR was performed in one step (single tube) in a 25 μl final volume using introns Maxime PCR PreMix Kit (I-Taq) (Korea). An initial preheating at 94°C for 5 min was followed by denaturation at 94°C for 30 Sec, annealing at 63°C for 30 Sec, and extension at 72°C for 50 Sec for 40 cycles followed by a final extension at 72°C for 5 min performed on a thermocycler. After PCR amplification, gel electrophoresis was performed in a 2% agarose gel at 130 V for 30 min to detect the amplified regions of DNA, and agarose gels were exposed under UV light in gel documentation system. Interpretation was done by comparing bands to the expected product size (wild type CALR: 357 bp, CALR type 1 mutation: 302 bp, and CALR type 2 mutation: 272 bp).Statistical analysis: The statistical analysis of the results was done using the SPSS (vs. 23) statistical software. The Chi-Squared test was used to compare the frequencies of the categorical variables. A value of p<0.05 was considered statistically significant.
3. Result
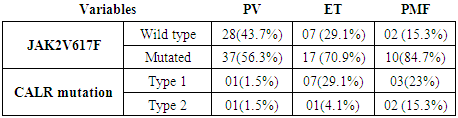
- Among the participants, the total JAK2V617f negative mutation was 37 (36.6%), in which 4/37 (10.8%) was found to be CALR type 1 mutation and 11/37 (29.7%) with CALR mutation type 2. From the 37 patients tested, CALR mutations were detected in 15 patients (40.5%), 8 patients (53.3%) of CLAR positive individuals, were diagnosed with ET, 5/15 (33.4%) diagnosed with MF and 2/15 (13.3%) diagnosed with PRV (table 1).
|
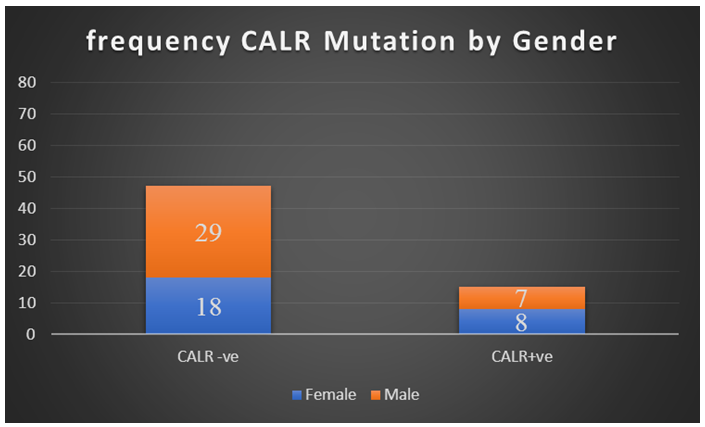 | Figure 1 |
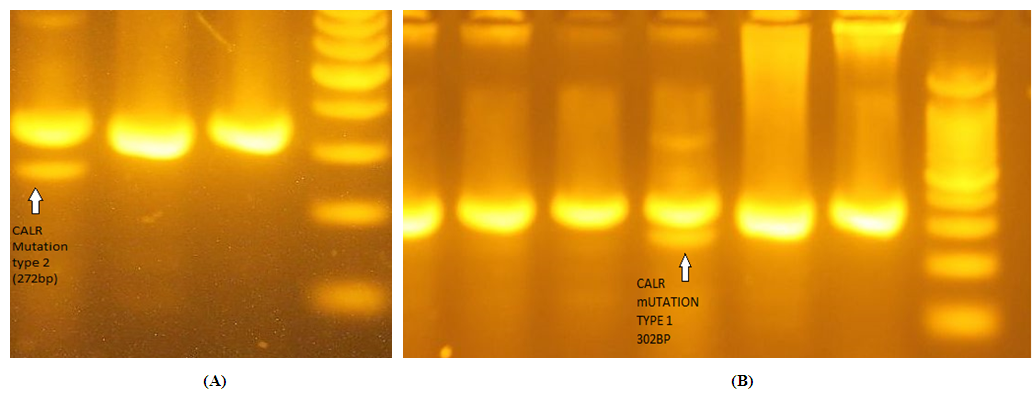 | Figure 2. (A) CALR mutation type 2 (272bp), the two thick bands represent normal samples of 357pb of CALR gene (B) CALR mutation type 1 (302bp) |
4. Discussion
|
ACKNOWLEDGMENTS
- The authors are most grateful to the RICK staff for kind assistance and appreciate all the patients who participate in our study. Our gratitude to institute of endemic disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML