-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2016; 5(1): 10-16
doi:10.5923/j.rct.20160501.02

Evaluation of OPN Level and VDR Gene Polymorphism in Patients with Hepatocellular Carcinoma
Eman M. I. Youssef1, 2, Fatma Saffeyeldin Mohamed3, Awatif Elmohamady Edreis4, 5, Wael F. Sedik2, 6, Mohamd A. Alblihed7, Amal A. Soliman8, 9, Rehab M. Elsaid Tash10, Nada Hassan M. Ahmed2, Marwa M. Hassan11, Mona El-Fedawy El-Saied11, Mirhan M. Elkady12, Heba Elhakeem12
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Medical Biochemistry, College of Medicine, Taif University, KSA
3Department of Tropical Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Tropical Medicine, Faculty of Medicine, Tanta University, Cairo, Egypt
5Department of Internal Medicine, College of Medicine, Taif University, KSA
6Department of Medical Biochemistry, Faculty of Medicine, Minia University, Egypt
7Department of Immunology and Endocrinology, College of Medicine, Taif University, KSA
8Department of Clinical Pathology, Faculty of Medicine, Menoufyia University, Egypt
9Department of Clinical Pathology, College of Medicine, Taif University, KSA
10Department of Medical Microbiology and Immunology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
11Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
12Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Eman M. I. Youssef, Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hepatocellular carcinoma (HCC) is considered one of the major causes of death. Markers of HCC have become helpful in screening, diagnosis and follow-up of cases. Osteopontin (OPN) is overexpressed in a variety of human tumors, including carcinomas of stomach, breast, prostate, lung, colon, and liver. Plasma level of OPN could be used as a biomarker for HCC. A variety of candidate genes has been identified, including the human vitamin D receptor (VDR). VDR is a product of the single chromosomal gene. Many single nucleotides polymorphisms (SNPs) have been identified in VDR gene, including the Bsm I (G/A) polymorphism. Objectives: To determine the plasma level of OPN and study the VDR Bsm I (G/A) gene polymorphism in HCC. Methods: Thirty-five HCC patients on top of HCV and 45 healthy controls were subjected to routine laboratory investigations including liver function tests and serum hepatitis markers. Both groups were tested for OPN level in the plasma of the studied subjects by ELISA and VDR Bsm Ι (G/A) genotypes by Real-Time PCR. Results: OPN levels were significantly elevated in patients with HCC in comparison to control group. On the other hand, no significant differences in VDR Bsm Ι (G/A) genotypes or alleles frequencies could be identified between the group of HCC and the control one. Conclusions: The results of this study suggest that plasma OPN level appears to be additional tumor marker for HCC. Nevertheless, VDR Bsm Ι (G/A) gene polymorphisms do not exhibit a significant influence on HCC susceptibility.
Keywords: OPN, Tumor marker, Gene Polymorphism, Vitamin D receptor (VDR), VDR Bsm Ι (G/A) genotypes, Single nucleotides polymorphisms (SNPs), HCC
Cite this paper: Eman M. I. Youssef, Fatma Saffeyeldin Mohamed, Awatif Elmohamady Edreis, Wael F. Sedik, Mohamd A. Alblihed, Amal A. Soliman, Rehab M. Elsaid Tash, Nada Hassan M. Ahmed, Marwa M. Hassan, Mona El-Fedawy El-Saied, Mirhan M. Elkady, Heba Elhakeem, Evaluation of OPN Level and VDR Gene Polymorphism in Patients with Hepatocellular Carcinoma, Research In Cancer and Tumor, Vol. 5 No. 1, 2016, pp. 10-16. doi: 10.5923/j.rct.20160501.02.
1. Introduction
- Hepatitis C virus (HCV) considered one of the major cause of liver disease, it affects nearly 300 million people worldwide, and it is considered the most common cause of chronic liver disease. Although HCV infection is often asymptomatic, it is prone to progress to cirrhosis and hepatocellular carcinoma (HCC) [1]. HCC is the fifth most common cancer worldwide [2]. The prognosis of HCC patients is generally very poor, most studies reported a five-year survival rate of less than 5% in symptomatic HCC patients [3]. Furthermore, these tumors have been shown to be quite resistant to radiotherapy or chemotherapy, and long-term survival of patients occurred only with small asymptomatic HCCs [4]. Early detection of patients with HCC is attractive because it gives a better prognosis as HCC tends to grow slowly and stay confined to the liver [5]. And also, early detection of HCC opens doors for various effective treatments such as surgical resection, radiofrequency ablation, and transplantation, which can subsequently lead to long-term survivals in a great number of HCC patients [6, 7].Osteopontin (OPN) is a glyco-phosphoprotein that is expressed and secreted by several cell types, including bone, teeth, kidney, activated macrophages, leukocytes, activated T lymphocytes, extracellular fluids, at sites of inflammation, and in the extracellular matrix of mineralized tissues and cancer cells [8]. OPN interacts with a variety of cell surface receptors, including several integrins and CD44. Binding of OPN to these cell surface receptors stimulates cell adhesion, migration, and specific signaling functions. OPN is present in elevated levels in the blood and plasma of some patients with metastatic cancers [9, 10]. Overexpression of OPN has been found in many cancers; including breast [11], prostate [12], colon [13], liver [14], ovarian [15], brain [16] and lung cancers [17]. OPN is highly expressed in HCC [18, 19]. In HCC, there is an elevated expression of OPN at mRNA levels and it has correlated with metastasis and poor prognosis, suggesting that OPN might be deemed as a useful molecular marker for predicting the prognosis of HCC [20].Vitamin D receptor (VDR) is a 427-amino acid protein with molecular mass of 48.3 kD and it is a member of the nuclear receptor superfamily of ligand-inducible transcription regulatory factors including the steroid and thyroid hormone receptors which are involved in many physiological processes, including regulation of calcium homeostasis, cell growth and differentiation, detoxification of xenobiotic and modulation of adaptive and innate immunity and it has major anti-cancer action in the form of anti-proliferative and apoptosis [21]. The VDR protein contains a zinc-finger DNA-binding and transcriptional activation domain and a ligand binding domain. VDR is found in all target tissues of vitamin D such as kidney, liver, and bone. The human VDR is a product of a chromosomal gene which locates on chromosome 12 at 12q13-14 [22]. Many single nucleotides polymorphisms have been identified in VDR gene including a Fok 1 restriction fragment length polymorphism in exon 2, Bsm 1 and Apa1 polymorphisms in the intron between exons 8 and 9, Taq 1 in exon 9. The Apa1 and the Bsm1 polymorphisms of the VDR gene are considered to be silent single nucleotide polymorphisms (SNPs), these polymorphisms do not change the amino acid sequence of the encoded protein. However, they may affect gene expression through regulation of mRNA stability [23, 24]. Bsm Ι (G/A) polymorphism of VDR gene has been explored in cancers of epithelial origin such as breast, ovarian, prostate, lung, skin cancers and HCC [25, 26]. In hepatocellular carcinoma, it was found that carriage of the (GG) genotype of Bsm Ι (G/A) was strongly associated with the occurrence of HCC in patients with liver cirrhosis [27].
2. Patients and Methods
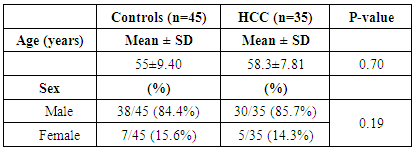
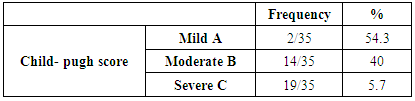
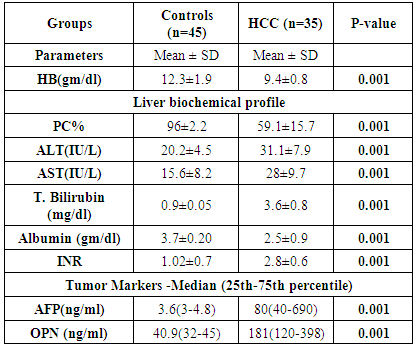
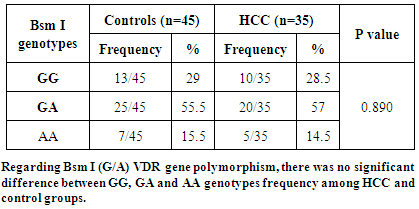
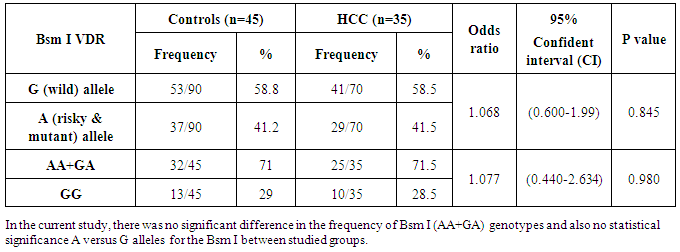
- Subjects and Controls: This study was conducted on 35 patients with HCC, diagnosed by ultrasound and confirmed with triphasic CT and alpha-fetoprotein; they were selected from the outpatient clinic of Tropical and Internal Medicine Departments of Al-Zaharaa University Hospital, Cairo, Egypt and 45 apparently healthy subjects, age and sex matched, having no acute or chronic illness and taking no medications were taken as control group. Informed oral consents were taken from all participants in this study. The patients were included according to the following criteria: Inclusion criteria: Patients aged 40 to 70 years old and seropositive for HCV antibodies. Exclusion criteria: Patients had any other tumor than HCC, patients with any bony lesions or inflammatory diseases or patients with poorly controlled diabetes mellitus or hypertension were excluded from the study. All candidates (HCC patients and control subjects) were subjected to: Demographic data: age and sex, full history taking, complete clinical examination and routine laboratory investigations including: CBC, liver function tests [serum alanine transaminase (ALT), aspartate transaminase (AST), albumin and total bilirubin, and prothrombin time (INR)] and viral markers including, HCV antibodies by ELISA technique. Assessment of liver disease severity was done by calculation of Child-Pugh score [28, 29]. Serum alpha-fetoprotein (AFP) using enzyme-linked immunosorbent assay (ELISA) technique [30]. Determination of osteopontin (OPN) levels by ELISA and VDR Bsm I (G/A) gene polymorphism by Real-time PCR. Sample collection: 7 ml of venous blood samples were withdrawn from each subject under complete aseptic conditions and divided as follows: 2 ml were collected in a sterile ethylene-di-amine-tetra-acetic acid (EDTA) vacutainer tubes and stored at –20°C to be used for assay of VDR Bsm I (G/A) gene polymorphism. 3ml were collected in plain tubes and left for 10 minutes to clot and then centrifuged at 3000 rpm for 5 minutes. The sera were then separated for routine laboratory investigations. The last 2ml were collected in a plastic tube containing EDTA as an anticoagulant, centrifuged at 1000 g for 15 minutes within 30 minutes of collection, and isolated plasma sample was stored at -20°C until measurements of OPN levels. Repeated freeze –thaw cycles were avoided.Determination of plasma osteopontin by ELISA: Plasma OPN levels were measured using the quantitative sandwich enzyme immunoassay technique (ELISA) purchased from plasma OPN using recombinant human OPN ELISA kit lot # DOST00(R&D Systems, Inc. 614 McKinley Place NE Minneapolis, MN 55413 United States of America), according to the manufacturer’s instructions. Determination of Bsm Ι vitamin D receptor gene polymorphism by Real-time PCR: DNA extraction from peripheral blood leucocytes using Favorgen Favorprep Genomic DNA blood Mini kit. The extracted DNA was then amplified according to the protocol proposed by [31]. Genotype analysis was determined by real-time PCR. DNA Extraction: DNA extraction was done using Favorgen Favorprep Genomic DNA Blood Mini kit (from FAVOREGEN Biotech Corp), No.37, Nong-KeRd., Ping-Tung 908, Taiwan. The extraction of DNA from whole blood encompassed the lysis of proteins, nucleases and contaminants by proteinase K enzyme with the lysis buffer. DNA purification was carried out using FABG Spin Columns. The lysate buffering conditions allowed optimal binding of the DNA to the FABG column membrane as soon as the sample was loaded onto the Spin Column. DNA was adsorbed onto the Favorgen silica-gel membrane during a brief centrifugation. Salt and pH conditions in the lysate ensured that proteins and other contaminants, which could inhibit PCR were not retained on the membrane. DNA bound to the membrane was washed with two different wash buffers in two centrifugation steps to improve the purity of the eluted DNA. Purified DNA was eluted from the Favorgen spin column in a concentrated form in the elution buffer. Amplification and Real-time PCR allelic discrimination assays: Real-Time PCR with sequence-specific primers was used to define the Bsm I (G/A) VDR gene SNP (rs1544410) in the intron between exons 8 and 9 at position 60890 base pair. Real-time PCR allelic discrimination assays were designed using Taq-Man SNP Genotyping Assays (Applied Biosystems). The TaqMan SNP Genotyping Assay: During PCR, the following steps occur; each TaqMan Minor groove binder (MGB) probe anneals specifically to its complementary sequence between the forward and reverse primer sites. When the oligonucleotide probe is intact, the proximity of the reporter dye to the quencher dye results in quenching of the reporter fluorescence primarily by Forster-type energy transfer [32, 33]. AmpliTaq Gold DNA polymerase extends the primers bound to the template DNA. AmpliTaq Gold DNA polymerase cleaves only probes that are hybridized to the target. Cleavage separates the reporter dye from the quencher dye, which results in increased fluorescence by the reporter. The increase in fluorescence signal occurs when probes that have hybridized to the complementary sequence are cleaved. Thus, the fluorescence signal generated by PCR amplification indicates which alleles are present in the sample. Allelic Discrimination Plate Read and Analysis: After PCR amplification, an endpoint plate read was performed using an Applied Biosystems Real-Time PCR System. The Sequence Detection System (SDS) Software used the fluorescence measurements made during the plate read to plot fluorescence (Rn) values based on the signals from each well. The plotted fluorescence signals indicated which alleles were in each sample. Performing a plate read and analyzing the data from TaqMan SNP Genotyping Assays. Statistical Methods: Data obtained from the study were coded and entered using the software SPSS (Statistical Package for Social Science) Version 17. Parametric data were summarized using mean and SD, whereas nonparametric data were summarized as median and percentiles for quantitative variables, and frequency and percentages were used for qualitative variables. Comparison between groups was done using the Chi-square test and the Fischer exact test for a qualitative variable, t-test and non-parametric Mann-Whitney U test were used to compare two groups. The odds ratio (OR) and their 95% confidence intervals (CIs) were calculated to estimate the strength of the association between polymorphism genotype alleles and patients and controls. The correlation analysis was assessed using the Spearman coefficient of correlation. Receiver operator characteristic (ROC) curve was plotted to detect the best cutoff value for the diagnosis of HCC. P value < 0.05 was considered significant.
3. Results
|
|
|
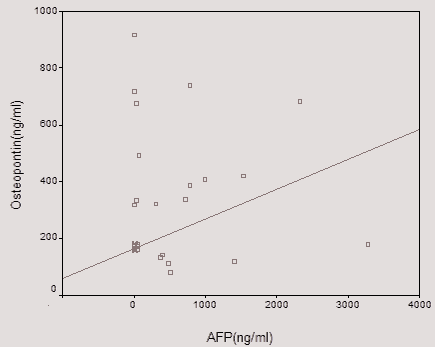 | Figure 1. Shows that there is direct significant correlation between plasma OPN level and serum AFP level of the patient as AFP level increases with increase of OPN level |
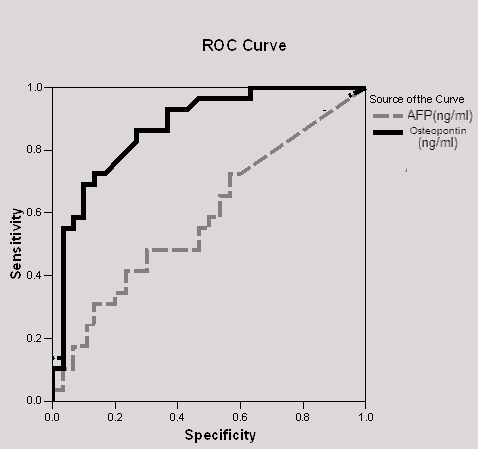 | Figure (2). ROC analysis of the studied tumor markers (OPN and AFP) in the diagnosis of HCC |
|
|
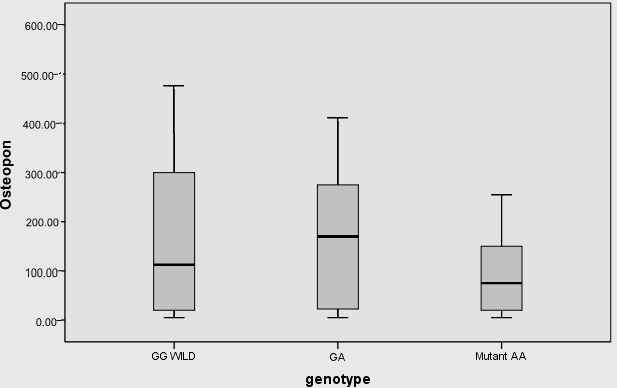 | Figure (3). Median of OPN levels among VDR Bsm Ι (G/A) genotypes, there was no significant difference in the median of OPN between VDR Bsm Ι (G/A) genotypes |
4. Discussion
- Hepatocellular carcinoma is the second most common cancer in men and the 6th most common cancer in women [34]. With advances in the understanding of tumor biology, along with the development of cellular and molecular techniques, the role of biomarkers related to early detection, invasiveness, metastasis, and recurrence has attracted a great deal of research interest resulting in discovery and utilization of several novel markers [35]. On analysis of the tumor biomarkers, regarding the results of the AFP in the studied groups it showed that serum level of AFP was significantly higher in HCC patients than controls (p-value =0.001). These findings were in agreement with Gad et al. [36] who found a significantly higher sensitivity of AFP in Egyptian patients in comparison with Japanese patients for HCC diagnosis (99% versus 67% P < 0.001) for AFP level greater than 10ng/mL, with comparable specificity (75% versus 82%). Also, Di Bisceglie et al. [37] who found that elevated serum AFP level among patients with chronic HCV.By studying plasma OPN level in the two groups we found that: significant elevation of plasma OPN levels in HCC patients than the lower levels in normal controls. In addition, El-Din Bessa et al. [38], Gad et al. [36], Abu El Makarem et al. [39] and Zhang et al. [40], found that the median plasma OPN level was significantly higher in the HCC group than in the normal control group. Also these results are in agreement with those of Hui et al. [41] who found that the median plasma OPN level of patients with HCC was 176.90 ng/ml (range 13.73–780.00 ng/ml), which was significantly higher than that of healthy volunteers 63.74 ng/ml (range 12.20–122.32 ng/ml). And Zhao et al. [42] who found that plasma OPN level in HCC group 13.38 (range from 9.2-23.6ng/ml) were significantly higher than in patients with cirrhosis 4.5 (range from 3.15-6.43ng/ml) than the control group 3.2 (range from 2.6-4.9ng/ml). Kim et al. [6] showed that median plasma OPN level in the chronic liver disease group was significantly higher than that of healthy controls, a possible proinflammatory role of OPN in chronic hepatitis and cirrhosis was suggested. Plasma OPN levels were not significantly affected by age or sex in the studied subjects, these results were in agreement with those of Kim et al [6] and Zhao et al. [42]. Both found that there were no differences in OPN levels between males and females.This study found that there was a significant positive correlation between OPN and AFP levels and it was in agreement with Hui et al. [41] who found that the plasma OPN level was correlated with the serum AFP concentration but it wasn’t coinciding with Kim et al. [6] who found that the correlation between plasma OPN and serum AFP was insignificant.In the present study, the ROC curve was made to detect a diagnostic accuracy of plasma OPN, the sensitivity, specificity, of OPN for selective detection of HCC group were 95% and 90% respectively at a cut-off level of 266 ng/ml with accuracy 93%. Our previous results were in agreement with a study conducted by Kim et al. [6] who reported diagnostic sensitivity and specificity of OPN for HCC group over non-HCC group (CLD group and healthy control) to be 93.5% and 84.2%, respectively, at a cut-off level of 552.9ng/ml. In agreement with our results, Keddeas and Abo-shady [43] found that the plasma OPN level was significantly higher in 40 patients with HCC compared with control participants (P < 0.01). OPN at the best cut-off value (325.5 ng/ml) had a sensitivity of 87.5% and specificity of 80% for detection of HCC cases (area under the curve = 0.876). Also in agreement with a recent study done by Sufen et al. [44] who reported that the OPN and AFP levels were subsequently measured in 312 plasma samples collected from 131 HCC patients, 76 cirrhosis patients, 52 chronic hepatitis C (CHC) patients. When HCV-associated HCCs were compared with HCV-associated cirrhosis, the AUC for AFP was (0.64), whereas OPN had an even higher AUC (0.80).The sensitivity and specificity of plasma OPN levels in HCC were 95% and90 % respectively, at a cut-off value of 266ng/ml. For AFP at a cut-off value 20 ng/mL; the values of sensitivity and specificity were 46% and 88% respectively. Results of our study were in accordance with the study done by Abu El Makarem et al. [39]. They found that the median plasma OPN level was significantly higher in the HCC group than in the cirrhotic patient group or in the normal control group (p-value < 0.001). The diagnostic efficacy of OPN was superior to AFP in terms of sensitivity, specificity. The sensitivity, specificity of plasma OPN levels in HCC patients relative to the CLD group were 97.67% and 100% respectively, at a cut-off value of 300ng/ml.VDR gene has been explored in cancers of epithelial origin such as breast, ovarian, prostate, lung, skin cancers and HCC [25, 26]. Even though some studies did not detect associations between the VDR polymorphisms and these diseases, e.g. Gsur et al. [45] in prostate cancer and Dunning et al. [46] in breast cancer, the majority of the authors found a significant association between the VDR polymorphisms and cancer [47]. In particular, carriage of the B BsmI A>G (B/b) allele has been described to exert a protective effect in prostate cancer [45], malignant melanoma [48] and breast cancer [49]. In the present study, there was no significant difference in VDR Bsm Ι (G/A) genotypes and alleles frequency among HCC and the control group (P = 0.980 and 0.845 respectively). Also, there was no significant difference between VDR Bsm Ι genotypes regarding the median level of OPN in HCC patients. Similarly, Xing et al. [50] did not find significant association between VDR Bsm Ι (G/A) polymorphism and hepatocellular carcinoma susceptibility in Chinese patients with chronic HBV infection, where percent of G allele was 50.57% in cases and 51.03% in controls while an allele was 49.43% in cases and 48.97% in controls (P =0.84). Falleti et al. [27] found in contrary that HCC was associated with the b allele of the BsmI A>G (B/b) polymorphism and that patients with HCC with liver cirrhosis were more likely to carry the b/b genotype compared with the B/B + B/b. Although many factors may be account for the discrepancies among these studies, the ethnic difference should be predominately considered since Fan et al. [51] found the distribution of FokI, BsmI, ApaI, and TaqI gene types significantly differed between Chinese healthy controls and Caucasian healthy controls.In the view of current data, the present study provides an evidence that the plasma OPN level was clearly elevated in HCC patients. Therefore, plasma OPN levels might be helpful for the diagnosis of HCC and serve as a prognostic indicator for patients with HCC. Additionally, OPN is superior to the traditional tumor biomarker as AFP. The results obtained in this study will be valuable for the future application of plasma OPN level as a routine biomarker for clinical prediction of the recurrence, metastasis and prognosis in HCC. On the opposite side, the data of this study suggests that VDR Bsm I (G/A) gene polymorphism does not exhibit a significant influence on HCC. The Greater number of patients are recommended to gain greater insight into the potential usefulness of OPN in patients with HCC especially those with normal levels of AFP. A larger sample size is recommended using a greater number of subjects to avoid statistical interference and bias. Combined studies of different markers are recommended as AFP, AFP-L3, PIVKA and DCP with OPN to increase the efficacy for diagnosis of HCC. Also, Multigenetic studies are recommended on genetic susceptibility to HCC.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML