-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2015; 4(1): 7-14
doi:10.5923/j.rct.20150401.02
The Potential Role of Angiopoietin-2 as a Diagnostic Tumor Marker for Hepatocellular Carcinoma
Eman M. I. Youssef1, Haneya A. A. Ali2, Amany M. Tawfik2, Nashwa El-Khouly3, 4
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Microbiology & Immunology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Internal Medicine, Faculty of Medicine, Taibah University, KSA
Correspondence to: Eman M. I. Youssef, Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and a global health problem because of its increasing prevalence worldwide and its poor prognosis. In fact, the incidence of HCC has increased sharply in the last 5–10 years, with an especially high incidence in Egypt resulting from chronic HBV and HCV infections due to agricultural pesticide and dietary aflatoxin exposures. Therefore, new and more specific markers for HCC are critically needed. Angiopoietin-2 (Ang-2) is over expressed in HCC, a highly vascularized tumor, and associated with portal infiltration, micro-vessel density, recurrence of HCC and decreased survival. Objectives:The aim of this study was carried out to evaluate the role of serum Ang-2 and to assess its sensitivity and specificity as a diagnostic tumor marker in HCC patients with liver cirrhosis.SubjectsandMethods: This study was conducted on 72 subjects. Patients divided into two groups: Group I comprised 25 patients with cirrhosis; group II comprised 22 patients with HCC. Both groups were compared to 25 (age and sex matched) healthy persons (group III). Following clinical assessment and radiological investigations, viral hepatitis markers, serum assay of Alpha-fetoprotein (AFP) and Ang-2 were performed using ELISA technique to all participants. Results: Serum Ang-2 was significantly higher in HCC patients compared to liver cirrhotic patients and normal controls. The sensitivity and specificity in diagnosis of HCC were 96% and 76% respectively at best cutoff points of 5360 pg /ml with accuracy 90%.Conclusions: Higher levels of serum Ang-2 in HCC patients than controls propose it as a potential tumor marker for diagnosis of this disease.
Keywords: Hepatocellular carcinoma (HCC), Alpha-fetoprotein (AFP), Angiopoietin-2 (Ang-2)
Cite this paper: Eman M. I. Youssef, Haneya A. A. Ali, Amany M. Tawfik, Nashwa El-Khouly, The Potential Role of Angiopoietin-2 as a Diagnostic Tumor Marker for Hepatocellular Carcinoma, Research In Cancer and Tumor, Vol. 4 No. 1, 2015, pp. 7-14. doi: 10.5923/j.rct.20150401.02.
Article Outline
1. Introduction
- Hepatocellular carcinoma (HCC) is rapidly reduces quality of life and responsible for a large proportion of cancer deaths worldwide. [1, 2]. This cancer varies widely in incidence throughout the world, with rising incidence in Egypt [3, 4]. Infections with hepatitis B or C virus, as well as dietary aflatoxin exposure are considered major risk factors for the progression to liver cirrhosis and HCC [5-7]. Egypt has the highest prevalence of HCV in the world [3]. There are no specific symptoms of HCC, making early diagnosis and detection of the disease difficult [8-10]. When HCC presents with specific clinical symptoms, the tumor is typically very far advanced [11, 12]. Alpha-fetoprotein (AFP) is the most validated serological diagnostic marker for HCC [4, 8]. However, its diagnostic value is more and more questioned. AFP levels drop markedly at birth, but are elevated in patients with HCC, cirrhosis, chronic hepatitis, liver necrosis, pregnancy, or gonadal tumors [13]. The use of AFP as a diagnostic and screening test for HCC is limited. This is in part due to the poor performance of available tumor markers leading to delay in diagnosis [14]. Therefore, new and more specific markers for early detection of HCC are critically needed to improve the survival of affected patients [15, 16].Simultaneously, angiogenesis is the process of formation of new capillaries from preexisting blood vessels [17, 18], it represents an essential component of embryogenesis, normal physiological growth, repair, tumor expansion and progression of cancer, correlating with the metastatic potential of tumor cells [19]. It is stimulated to provide oxygen and nutrients to injured tissue; unfortunately, chronic damage leading to capillarization of hepatic sinusoids, which restricts the blood supply, exacerbating tissue injury, fibrogenesis, and angiogenesis [20, 21]. This sequence of events appears to govern the progression of CHC to cirrhosis and HCC [22]. One of the most significant signaling pathways in pathological angiogenesis and HCC is the angiopoietin/Tie2 system [23]. The angiopoietins serve as ligands for the endothelium-specific receptor tyrosine kinases Tie1 and Tie2, which comprise 4 structurally related proteins, termed angiopoietin (Ang)-1, Ang-2, Ang-3 and Ang-4 [20, 21]. Ang-2 (Ang-2; ANGPT2) is a member of the angiopoietin protein family (angiopoietin-1, -2, -3, and -4 which plays an important role in the development and maintenance of blood vessels and the lymphatic system [24-26]. The gene for human angiopoietin-2 maps to chromosome 8p23 and the mature protein contains 496 amino acid residues. After signal sequence processing and maturation, Ang-2 becomes 478 amino acids long (from 19 to 496). In addition, several N-terminal truncated forms arise due to alternative splicing, including Ang-2 443, expressed by primary endothelial cells and tumor tissues [27, 28]. High serum Ang-2 values are found in patients with inflammatory conditions as chronic HCV infection [29-31], inflammatory bowel disease and sepsis [32, 33]. Also production of Ang-2 has been implicated in tumor development in human gastric and colon cancers [34], human prostate carcinoma, and human breast cancers [35]. Ang-2 is overexpressed in HCC and associated with portal infiltration, micro-vessel density, recurrence of HCC and decreased survival [19, 29]. Recent studies reported high serum Ang-2 values in patients with HCC suggesting that Ang-2 might represent a useful marker for HCC and a complementary diagnostic tool [36, 37].
2. Subjects and Methods
2.1. Subjects
- The study included seventy two subjects; their ages ranged 40 - 69 years. Patients were attending the outpatient clinic of Internal Medicine Department of Al-Zaharaa University Hospital, Cairo, Egypt, from September 2013 till May 2014. According to the results of abdominal ultrasound (US), abdominal triphasic CT scan and laboratory investigation including alpha fetoprotein, the patients were classified into two groups as follows: Group I: included twenty two patients HCC on top of chronic HCV induced cirrhosis. They were (15 males and 7 females), their ages ranged between 45 and 69 years with a mean ±SD (57.8±5.9 years). Group II: included twenty five chronic hepatitis C virus (CHCV) patients with liver cirrhosis and they were 16 males and 9 females and their ages ranged between 40 and 64 years old with a mean ±SD (54.7±6.2 years). Exclusion criteria: The patients with known history of autoimmune diseases, diabetes mellitus, hypertension, renal or cardiopulmonary diseases, chronic inflammatory disease and a history of cancer other than HCC and with metastatic HCC, as well as patients with chronic liver disease other than chronic HCV were excluded from this study. In addition to twenty five healthy individuals with matched age and sex to the patients were included into the study who served as a control group (Group III), they were 18 males and 7 females their ages ranged between 43 and 60 years with a mean ±SD (49.8±7.5 years). They were completely free (clinically, with normal laboratory findings, negative viral hepatitis markers and normal abdominal ultrasonographic findings). This study was performed after obtaining informed consent from all participating subjects enrolled in the study.
2.2. Methods
- All patients and control were subjected to the following: 1- Full history taking and complete clinical examination with stress on jaundice, hepatomegaly, ascites, splenomegaly, lower limb oedema, and encephalopathy. 2- Abdominal ultrasound, to assess the echo pattern and size of the liver and the presence of ascites, the size of spleen or any other abnormalities. 3- Triphasic computed tomography (CT) scan. Laboratory investigation: Liver function tests in the form of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, complete blood picture, prothrombin concentration (PT), ESR and hepatitis viral markers including HCV antibody and HBV surface antigen. Determination of HBV surface antigen (HBsAg) was carried out by ELISA, one-step incubation, double antibody sandwich principle and Anti-HCV employs solid phase, indirect ELISA method for detection of antibodies to HCV in two-step incubation procedure. Polystyrene microwell strips are pre-coated with recombinant, highly immunoreactive antigens corresponding to the core and the non-structural regions of HCV (Third generation HCV ELISA), and both kits were supplied from Wkea Med Supplies Corp., Chenguang Gardon, Qianjin Street, Changchun 130012 China.Tumor markers: A 15 ml blood sample was drawn from each subject immediately after diagnosis. Blood samples were centrifuged at 2200 G/min for 10 minutes and serum stored at –70°C until tested for AFP and Ang-2. Serum AFP was measured using DRG® AFP ELISA Kit (Catalog number: EIA-1468) by Kit supplied from DRG International Inc., USA.
2.3. Detection of Human Angiopoietin-2 by ELISA Method
- Quantitative determination of human angiopoietin 2 (ANG-2) concentrations in serum by Human enzyme-linked immunoassay Ang-2 (ELISA) kits (Biosource International, USA), (Catalog number: MBS702275), according to the manufacturer’s instructions, this assay employs the quantitative sandwich enzyme immunoassay technique. Antibody specific for ANG-2 has been pre-coated onto a micro-plate. Standards and samples are pipetted into the wells and any ANG-2 present is bound by the immobilized antibody. Optical densities were measured at 450 nm using a micro-plate reader set to 450 nm. The concentrations were calculated from the standard curve generated by a four parameter logistic (4-PL) curve-fitting programme.
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the statistical package for social science (SPSS) version 20. The quantitative data were presented as mean, standard deviations and ranges. The comparison between the studied groups were done by using One Way Analysis of Variance (ANOVA) followed by post hoc analysis – least significant difference (LSD) test. The receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity and accuracy. The confidence interval was set to 95% and the margin of error accepted was adjusted to 5%. So, the p-value was considered insignificant at the level of > 0.05, significant at the level of < 0.05 and highly significant at the level of < 0.01.
3. Results
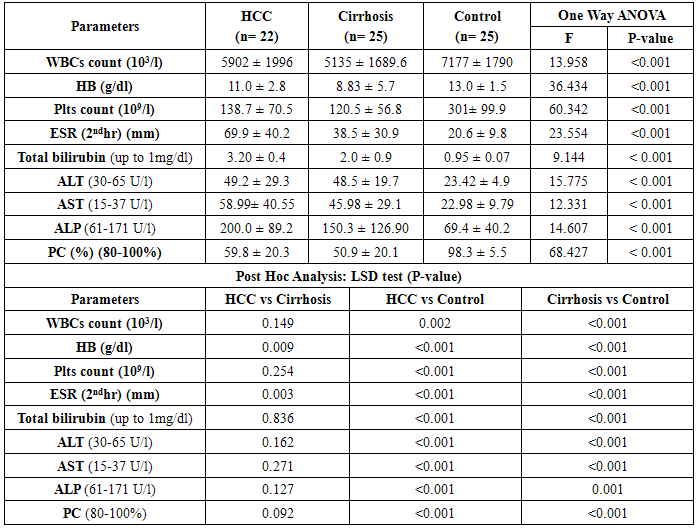
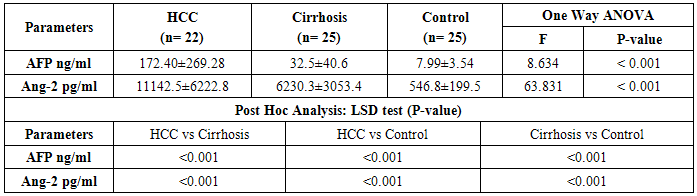
- This study included 47 Egyptian patients and 25 healthy age and sex matched control subjects. Thirty six (77%) of our patients were HCV-antibody +ve and none of them were positive for HBsAg. The results obtained are presented in tables (1-3) and figure (1). Detailed laboratory data of all studied groups are shown in table (1).
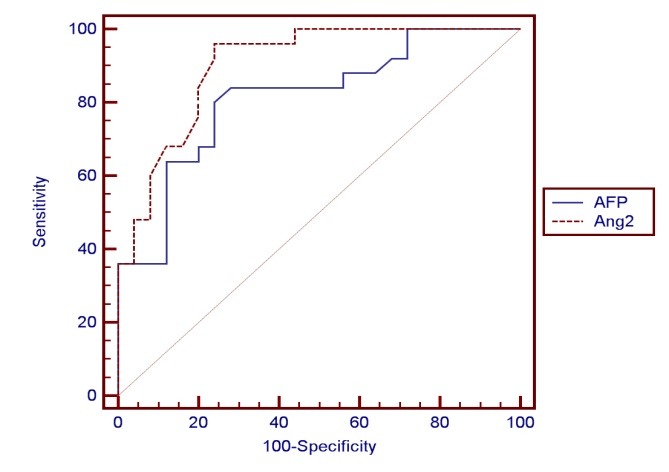 | Figure (1). ROC curve for AFP versus Ang – 2 in diagnosis of HCC |
|
|
|
4. Discussion
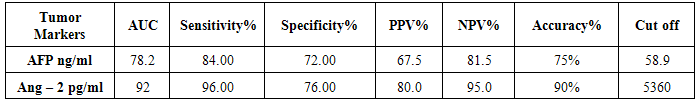
- HCC is a common problem worldwide which ranks the 5th and 7th most common cancer among men and women respectively [38, 39]. The main aim of this study is to study the role of serum Ang-2 levels in HCC Egyptian patients, to compare all groups with each other and with the normal controls and to verify the possibility of using serum Ang-2 levels as a potential biomarker in diagnosis of HCC. In the current study, thirty six patients (77%) were selected to have anti-HCV sero-positivity due to its high prevalence rate in our country and this was in agreement with Nair et al. [40] who found that 80% of HCC patients were HCV positive. A total of 53% of our patients were cirrhotic and this was the major clinical risk factor for HCC development, this was in agreement with Blum et al. [41] who reported that 90% of patients developed HCC on top of cirrhosis. Concerning laboratory parameters in this study, most of the patients with cirrhosis and HCC had significantly lower hemoglobin levels and platelet counts (p≤0.001) in comparison to the healthy control group. Also, serum hemoglobin was lowest in patients with liver cirrhosis and HCC and highest in the control group which was statistically highly significant (p≤0.001). This difference could be explained by acute and chronic gastrointestinal blood loss, folate deficiency, hypersplenism, bone marrow suppression and the anemia of chronic disease. This is in agreement with Sakisaka et al. [42] who stated that although raised serum erythropiotin (EPO) levels may be present in up to 23 % of patients with HCC, elevations in hemoglobin concentration or packed cell volume are uncommon, and most patients are anemic at diagnosis because of other effects of the tumor.The total leucocyte count and platelet count were significantly lower in all groups compared with the control group (p≤0.001) which agreed with Pratt and Kaplan [43] who explained that leucopenia observed with liver cirrhosis is due to hypersplenism with splenic margination while thrombocytopenia is mainly caused by portal hypertension with attendant congestive splenomegaly. An enlarged spleen can result in temporary sequestration of up to 90% of the circulating platelet mass. Decreased thrombopoietin levels may also contribute to thrombocytopenia. In the present study we found that prothrombin concentration was statistically highly significantly low in patients with liver cirrhosis and HCC (p≤0.001) compared to controls. This could be explained by decreased synthesis of coagulation factors by the diseased liver. This agreed with what was reported by Tripod et al. [44] who observed poor utilization of vitamin k in advanced parenchymal liver disease.Additionally, a highly significant increase in AST, ALT, ALP, and total bilirubin were detected in cirrhosis and HCC groups compared to the control group. These tests were significantly worse in both patients group when compared to controls and this result comes in accordance with previous work by Sherlock and Dooley [45] who stated that, the liver function tests usually indicate the type and severity of liver injury. This study revealed no statistical significant difference in AST, ALT, ALP, and total bilirubin between both HCC and cirrhosis groups. Also, our results come in agreement with previous study by Kew [46] who declared that the conventional tests of hepatic function do not distinguish HCC from other hepatic masses or from cirrhosis, and so they contribute little to the diagnosis of the tumor. This is also in agreement with Sugiyama et al. [47] who found that liver function tests were significantly elevated in HCC patients (p≤0.001) when compared to chronic liver disease (CLD) patients. In agreement with our findings, HCC group had the highest values for various concurrently measured liver function tests significant higher values of AST/ALT, ALT, AST (each, p≤0.001) than cirrhotic patients. Also, this is in agreement with Abdel-Rahman et al. [13] who stated that liver function tests were significantly elevated (p≤0.001) in patients with HCC when compared to CLD. The current study showed that the results of mean serum levels of AFP in all studied groups it showed that HCC group had the highest level compared to other groups with statistically significant difference (p value≤0.001) between HCC versus other groups, a finding that came in agreement with previous studies of many authors Mittal et al. [48] and Guan et al. [49]. Also comparable to Gad et al. [50] found a significantly higher sensitivity of AFP in Egyptian patients in comparison with Japanese patients for HCC diagnosis (99% versus 67% P < 0.001). Similarly, Hussein et al. [51] showed a significant difference in AFP levels between patients with HCC (1040.29 ± 2315 ng/ml), chronic liver disease (8.47 ± 20.035 ng/ml) and controls (2.207 ± 1.44 ng/ml) (p < 0.05). AFP was higher in HCC patients when compared to patients with chronic liver disease with significant difference (p≤0.05). This was disagreement with Abdel-Rahman et al. [13] who stated that AFP levels are frequently normal in patients with small HCC and are not elevated in a significant portion in patients with early stage, potentially curable HCC. Also, in contrary with the study done by Huo et al. [14], who concluded that the serum AFP level was a weak diagnostic predictor in HCC patients.By studying Ang-2 levels in different groups, the serum Ang-2 had the highest mean levels in HCC group compared to other groups (p≤0.001), also significant difference between HCC versus control and cirrhotic groups (p≤0.001).This data comes in consistent with previous work by Badran et al. [29] who reported angiopoietin-2 serum levels were elevated in patients with liver cirrhosis and HCC patients compared to healthy controls (1671.6±284.2 vs1416.6±502.7 and 194.97±76.9 ug/ml P < 0.0001). Highest Levels were detected in HCC patients compared to cirrhosis patients (P < 0.0003). Additionally, Diaz-Sanchez et al. [36] who concluded that the Ang-2 seems to play an important role in the angiogenic processes of HCC and its serum levels are associated with tumor characteristics and invasive behavior. Another study done by Scholz et al. [52] who found a statistically highly significant elevation of Ang-2 serum levels in HCC patients when compared to cirrhotic patients and also reported that Ang-2 mRNA was expressed in most of HCC cryopreserved biopsies using in situ hybridization in addition to also agree with a previous work done by Hunter et al. [53] who reported a statistically highly significant elevation (p< 0.001) in the mean serum Ang-2 in HCC group (10855 ± 5321.92 pg/ml) when compared with both the control (480.67 ± 202.3 pg/ml) and cirrhosis (5578.33 ± 2928.21 pg/ml) groups. In the current study, the results revealed that there was a statistically highly significant elevation (p≤0.001) in the mean serum Ang-2 in cirrhosis group when compared to control group. These results are consistent with Scholz et al. [52] who reported a statistically highly significant elevation of Ang-2 serum levels in cirrhotic patients when compared to control subjects. Ang-2 is released in inflammatory conditions such as chronic HCV infection. In situ hybridization data had shown that Ang-2 mRNA expression was absent from hepatocytes of cirrhotic livers. Apart from endothelial cells of blood vessels, Ang-2 positive cells were found also within the connective tissue strands spanning between the portal tracts. Ang-2 expressing cells may include endothelial cells as suggested by their typical cellular morphology, as well as inflammatory and mesenchymal cells. These data may argue against inflammation as the sole reason for elevated Ang-2 levels in serum of cirrhotic patients and emerge the fibrosis process as an extra explanation.Concerning the correlation between laboratory data and serum Ang-2 levels, this study revealed that there was no significant correlation between all of the previous parameters and serum Ang-2 levels, among both cirrhosis and HCC groups, with the exception of indirect significant correlation between prothrombin concentration and serum Ang-2 in the cirrhosis group. Also, there was no correlation between serum Ang-2 and serum AFP in patients of both the cirrhosis and HCC groups in our study. This was in agreement with Scholz et al. [52] who reported no correlation between both markers in patients with HCC and in patients with cirrhosis.Further analysis of the data using ROC curve analysis and the corresponding area under the curve were attempted for the studied markers to investigate their diagnostic accuracy of HCC group demonstrating cut off values with their specificity, sensitivity, PPV and NPV. Serum levels of AFP had a sensitivity of 84% and specificity of 72% at cut off value 58.9 ng/ml, PPV 67.5%, NPV 81.5% and accuracy 75% between HCC and other groups. This result suggested that diagnostic accuracy of AFP in the diagnosis of HCC was poor as AUC was 78.2. These results were comparable to those of Gad et al. [50] showed cut off value for AFP similar to the present study, where they reported sensitivity of 86% and specificity of 78% at a cut off value of 10ng/ml for selective detection of HCC group over non HCC group. Also a study done by Hussein et al. [51] on an Egyptian population detected 89.8% sensitivity and 93.3% specificity for AFP at a cut off value of 7.7ng/ml. Our findings also are supported by Hernández et al. [54] constructed a ROC curve for AFP to diagnose HCC. The area under the curve was 0.846. They used a diagnostic threshold of 19.07ng/mL, at which sensitivity was 68.2%, specificity was 90%, PPV was 45.45% and NPV was 96.8%. ROC curve analysis was done in this study for serum Ang-2 to in HCC. It showed that Ang-2 sensitivity and specificity for diagnosis of HCC were 96% and 76% at a best cut off value 5360 pg/ml with area under the curve (AUC)= 92. The PPV and PNV were 80% and 95% respectively. The diagnostic accuracy of serum Ang-2 was 90%. So this lead to that best use or validity of Ang-2 was in diagnosis of HCC with high validity and overall accuracy. These results are consistent with Hunter et al. [53] who found that the cutoff level of Ang-2 for diagnosis of HCC was 8100 pg/ml, with a sensitivity and specificity of 70% and 80% respectively. Serum Ang-2 was significantly elevated in HCC patients with portal vein thrombosis than those without. There was a significant positive correlation between the number of hepatic focal lesions and the serum level of Ang-2 and concluded that the combined use of the two markers (AFP and Ang-2) led to an increase in the sensitivity of AFP from 53.3% to 83.3%. And also concluded that the serum Ang-2 is elevated in patients with cirrhosis and further elevated in patients with HCC, so its use as an independent tumor marker in the diagnosis of HCC is to be considered and the detection rates could increase when using both markers. On the other hand, our findings were in contrary with Badran et al. [29] who reported that serum angiopoetin levels was not different among HCC cases who had AFP>200 ng/ml and those who had lower AFP levels (1279.12±476.6 vs1493.98±507.7) P=0.059. So from our results and that of others we found that the serum angiopoietin-2 levels can be used in selective detection and a good diagnostic biomarker for HCC and it is relatively comparative to AFP and it is superior to it in diagnosis of HCC patients.
5. Conclusions
- Conclusively, serum Ang-2 levels were elevated in HCC patients than patients with liver cirrhosis and then normal controls. Also Ang-2 is relatively comparative to AFP in detection of HCC among high risk groups and superior to AFP. No significant correlation was found between the two markers in HCC and in the cirrhosis groups. Also it was found that when considering a cutoff level of AFP at 58.9 ng/dl with a sensitivity of 84% and a specificity of 72%. The cutoff value of Ang-2 for diagnosis of HCC in this study was 5360 pg/ml, with a sensitivity and specificity of 96% and 76% respectively. These results suggest that Ang-2 was a potential diagnostic tumor marker for HCC, especially among high-risk group of patients. This value extends beyond the traditional tumor biomarkers as AFP, as it possess good prognostic value. Although, AFP has to be considered ‘the golden standard’ for HCC serum markers for years, in the view of our data and that of others; the usefulness of AFP testing for the population at risk should be seriously questioned. Ang-2 levels appear to be an additional tumor biomarker for HCC detection especially among high risk group of patients. Our study have a limitation that we assessed the levels of AFP and the level of Ang-2 in a limited number of patients and should be higher than the number included in our study. Another important issue that limits the potential utility of Ang-2 levels as a specific biomarker for cancer is that Ang-2 level is also increased in a range of inflammatory syndromes. A screening program for early detection of HCC is recommended to all patients with liver cirrhosis by the use of tumor-markers and ultrasonography. Triphasic CT scan and/or liver biopsy may be needed to confirm the diagnosis. Therefore, further careful evaluation with one standardized assay system will be needed to gain greater insight into the potential usefulness of Ang-2 in patients with.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML