-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2015; 4(1): 1-6
doi:10.5923/j.rct.20150401.01
Fatty Acid Composition of Human Periprostatic Adipose Tissue from Argentine Patients and Its Relationship to Prostate Cancer and Benign Prostatic Hyperplasia
Valeria P. Careaga1, 2, Paula A. Sacca1, Osvaldo N. Mazza3, 4, Carlos Scorticati3, Gonzalo Vitagliano4, Sabrina Johanna Fletcher1, Marta S. Maier2, Juan C. Calvo1, 5
1Instituto de Biología y Medicina Experimental (IBYME), CONICET, Buenos Aires, Argentina
2UMYMFOR (CONICET-UBA), Departamento de Química Orgánica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Buenos Aires, Argentina
3Hospital de Clínicas “José de San Martín”, Cátedra de Urología,Facultad de Medicina, Universidad de Buenos Aires, Argentina
4Hospital Alemán, Servicio de Urología, Buenos Aires, Argentina
5Departamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, UBA, Ciudad Universitaria, Buenos Aires, Argentina
Correspondence to: Juan C. Calvo, Instituto de Biología y Medicina Experimental (IBYME), CONICET, Buenos Aires, Argentina.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
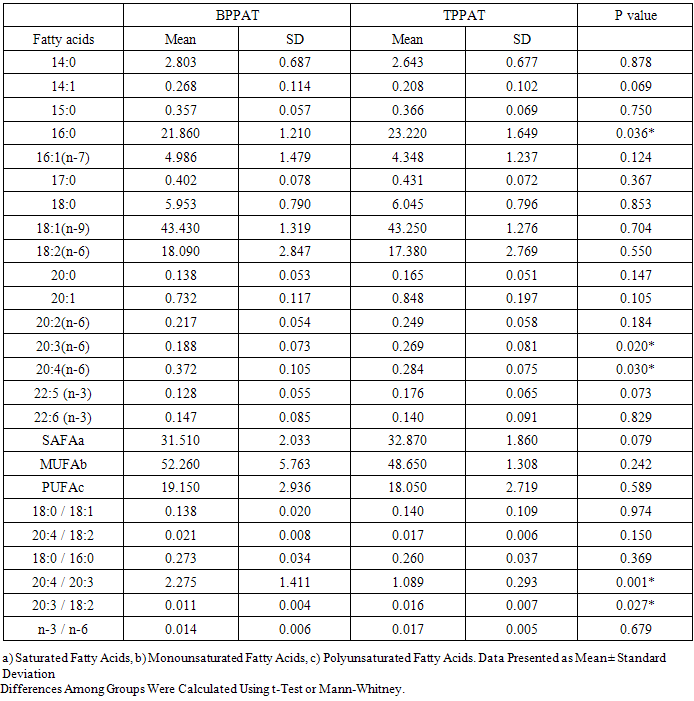
The aim of this study was to determine fatty acid composition in periprostatic adipose tissue (PPAT) of patients undergoing surgery for either prostatic cancer or benign prostatic hyperplasia (BPH). PPAT were obtained from 12 patients undergoing radical prostatectomy for clinically localized prostate tumors (TPPAT, age range 55-70 years) and 11 patients undergoing adenomectomy for BPH (BPPAT, age range 57-79 years). Fatty acid methyl esters of total lipids of PPAT were processed and then analyzed by gas chromatography-mass spectrometry. Quantitation was performed by comparing the percentage area of each FAME peak on the chromatogram with that of the internal standard of known weight, and expressed as percentage of total fatty acids. There were differences in fatty acid content of PPAT, with higher levels of palmitic acid (16:0; P = 0.036) and dihomo-gammalinolenic acid (20:3 n-6; P = 0.020) and lower levels of arachidonic acid (20:4 n-6; P = 0.030) in prostate cancer PPAT, along with a higher 20:4/20:3 (P = 0.001) and lower 20:3/18:2 (P = 0.027) fatty acid ratio in benign prostate hyperplasia PPAT. To the best of our knowledge, this study represents the first attempt at comparing periprostatic fat pad lipid composition in different prostate pathologies. Fatty acid analysis and lipidomics may be important tools to further understand events that occur in tumor microenvironment during prostate cancer disease.
Keywords: Prostate cancer, Benign prostate hyperplasia, Periprostatic adipose tissue, Fatty acids, Lipid composition, Lipidomics
Cite this paper: Valeria P. Careaga, Paula A. Sacca, Osvaldo N. Mazza, Carlos Scorticati, Gonzalo Vitagliano, Sabrina Johanna Fletcher, Marta S. Maier, Juan C. Calvo, Fatty Acid Composition of Human Periprostatic Adipose Tissue from Argentine Patients and Its Relationship to Prostate Cancer and Benign Prostatic Hyperplasia, Research In Cancer and Tumor, Vol. 4 No. 1, 2015, pp. 1-6. doi: 10.5923/j.rct.20150401.01.
Article Outline
1. Introduction
- According to statistics from the Argentine Ministry of Health, prostate cancer (PCa) is the most relevant cancer for males in Argentina and the second greatest cause of cancer mortality in men [1].There is an urgent need to find new and reliable biomarkers for this disease due to the limitations of currently available methods of detection. Lipids are known to be involved in many biological pathways and modification of cell lipid composition can lead to severe pathologies. Lipidomics is becoming an expanding field of research, and the increased volume of study in this area may well contribute to the discovery of new biomarkers for several diseases including cancer [2, 3].Epidemiological studies have reported on the effect of dietary fat on prostate cancer risk [4-6]. However, the mechanistic role of dietary fat in PCa remains ill defined. Dietary fat includes n-3 and n-6 polyunsaturated fatty acids (PUFAs), both of which play important roles in many human biological processes, including PCa [7]. Because humans cannot synthesize n-3 and n-6 PUFAs, they are considered essential fatty acids. All mammalian cells can interconvert the PUFAs within each series by elongation, desaturation, and retro conversion, but the two series are not interchangeable. n-3 and n-6 PUFAs can be metabolized by cyclooxygenase (COX) and lipoxygenases (LOX); the resulting eicosanoids have been implicated in the pathogenesis of a variety of human diseases, including cancer. n-6 derived prostaglandins (PGs), especially PGE2 derived from the metabolism of arachidonic acid (AA, 20:4 n-6), are strongly implicated in tumor growth and metastasis, inhibiting apoptosis and enhancing angiogenesis [8, 9].To date, very little is known about the cross-talk between adipose tissue and prostate cancer cells. Periprostatic adipose tissue (PPAT) surrounds the prostate gland and is part of tumor microenvironment. PPAT thickness has been associated with PCa aggressiveness [10] and high-grade PCa diagnosis [11] and furthermore, factors secreted by PPAT have been shown to induce a favorable microenvironment for PCa progression [12, 13]. Results from our laboratory have shown that PPAT derived factors could modulate disease progression in the early stages of PCa [14]. Recently, a gene expression signature study of PPAT suggested that the local environment could be allowing cancer progression in obese men exhibiting an anti-lipolytic and adipo/lipogenic gene expression profile [15].The aim of the present study was to evaluate if specific fatty acids in PPAT could modify PCa development. Despite the small number of patients analyzed, to our knowledge this is the first report on the analysis and comparison of fatty acid composition of PPAT in PCa and benign prostatic hyperplasia (BPH) patients.
2. Materials and Methods
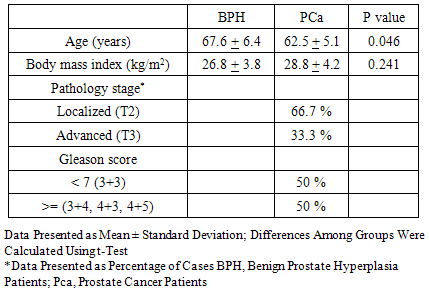
- Human PPAT was obtained from 23 patients attending the Urology Department, Hospital de Clínicas “José de San Martín”, Buenos Aires, Argentina. Twelve patients underwent radical prostatectomy for clinically localized prostate tumors (TPPAT, age range 55-70 years), and 11 patients underwent adenomectomy for BPH (BPPAT, age range 57-79 years). None of the patients had received preoperative therapy. Patients gave their written consent for tissue harvesting for this study and all experimental procedures were approved by IBYME (Instituto de Biología y Medicina Experimental) Ethics Committee.In each patient, once the anterior surface of the prostate had been surgically exposed, the fat tissue surrounding the superficial venous plexus of Santorini was dissected. A fatty, loose tissue surrounds this plexus on both sides of the inferior portion of the anterior surface of the prostate, and approximately 30 – 50 mg of this fat tissue was separated for further processing.Clinical features of the patients (age, body mass index) and pathological characteristics of the specimens used (Gleason score and pathological stage (pT)) are shown in Table 1. All BPH specimens showed histological epithelial and/or stromal cell hyperplasia but no malignant cells.
|
3. Results
- PPAT samples from 23 patients were analyzed. Patients either underwent radical prostatectomy for clinically localized prostate tumors (TPPAT, n=12) or had adenomectomy for BPH (BPPAT, n=11). PCa patients had a mean age of 62.5 ± 5.1 years, significantly lower than BPH patients: 67.6 ± 6.4 years (Table 1).BMI was slightly higher, though not statistically significant, in subjects with PCa (n=11, patient 12 without BMI data) compared to those in the BPH group (28.8 ± 4.2 kg/m2 vs 26.8 ± 3.8 kg/m2, P = 0.241). Both groups had a mean body mass index (BMI) that corresponded to being overweight. Patients were further separated into two groups based on their BMI according to WHO classification: normal (N) and overweight-obese. Overweight and obesity was detected in 73% of PCa patients (8 out of 11) and in 55% of patients with BPH (6 out of 11). Correlation between BMI and fatty acid composition could not be evaluated due to the low number of PCa patients with a normal BMI.
3.1. Fatty Acid Composition of Periprostatic Adipose Tissue
- TLC analysis of lipid fractions obtained from PPAT revealed the presence of triacylglycerides as the main component. Fatty acid composition of PPAT obtained from BPH and PCa patients is summarized in Table 2. Data corresponded to both individual fatty acids and to fatty acids grouped according to their saturation characteristics: saturated (SFA), monounsaturated (MUFA) or polyunsaturated (PUFA).
4. Discussion
- To our knowledge, this is the first study that clearly indicates a difference in fatty acid composition of PPAT between patients with benign prostate hyperplasia and PCa.The role of lipid metabolism has gained popularity in PCa research, and de novo lipogenesis is under intense investigation as a therapeutic target [18]. Modification of cell lipid composition can lead to severe pathologies. Lipid droplets (LD) are inducible organelles constitutively expressed in adipocytes. They are involved in neutral lipid storage and have roles in cell signaling, regulation of lipid metabolism, membrane trafficking and control of the synthesis and secretion of inflammatory mediators. Enzymes involved in eicosanoid synthesis (COX and LOX) are localized in LD and these are sites for eicosanoid generation in cells during inflammation and cancer [19]. We showed that palmitic acid (C16:0) levels were higher in TPPAT than in BPPAT, possibly associated with an increased expression of fatty acid synthase (FAS), which has emerged as a common phenotype to most human carcinomas [20]. Palmitic acid is the major fatty acid produced by de novo lipogenesis from acetyl CoA and malonyl CoA by FAS [21]. Evidence suggests that the expression of FAS mRNA and protein are upregulated in prostate tumor tissues, an event that occurs early in the development of prostate cancer [22]. Under normal conditions, de novo fatty acid biosynthesis and FAS expression occur constitutively at very low levels, since the requirement for fatty acids is sufficiently met by dietary intake [21]. On the other hand, immunohistochemical studies have reported that FAS protein is overexpressed in the majority of human malignancies and their preneoplastic lesions, including PCa [23]. This study has also shown lower levels of AA in TPPAT than in BPPAT. AA is the most important PUFA associated with membrane phospholipids. The metabolism of this fatty acid by either COX or LOX pathway generates eicosanoids, which have been implicated in cancer, and are considered important in tumor promotion and progression in PCa [9, 24]. Increased AA metabolism via the COX, and possibly LOX pathway, could explain the lower AA concentration in malignant tissue observed in the present study. Preliminary in vitro studies have shown that malignant prostatic tissue has a greatly increased capacity for eicosanoid synthesis from radiolabeled AA compared to benign tissue [24]. A lower concentration of AA (measured in the phospholipid fraction of both plasma and tissue) has been found in subjects with PCa vs BPH, due to an increased metabolism via the LOX and COX pathways, and thus producing higher concentrations of eicosanoids [25, 26]. Tissue AA is derived primarily from dietary linoleic acid. This conversion is regulated by delta-6-desaturase, an enzyme that catalyzes the first step in this metabolic pathway [27]. Delta-6-desaturase incorporates a double bond at the C-6 carbon of PUFAs and is rate limiting in the desaturation and elongation of LA to AA (n-6 family) and α-linolenic acid (ALA, 18:3 n-3) to eicosapentaenoic acid (EPA, 20:5 n-3) (n-3 family). Delta-6-desaturase converts LA to γ-linolenic acid (GLA, 18:3, n-6) and GLA is elongated to form DGLA that can then be converted to AA by the action of the enzyme delta-5-desaturase. AA is the precursor of two series of prostaglandins, thromboxanes and four series of leukotrienes (LTs). Similarly, ALA is converted to EPA by delta-6-desaturase and delta-5-desaturase. EPA is the precursor of three series of prostaglandins and five series of LTs and EPA can also be elongated to form docosahexaenoic acid (DHA).It is probable that the lower amounts of AA found in TPPAT in this study were due to the combination of both a decreased activity of delta-5-desaturase and an increased metabolism via the LOX and COX pathways. There was a higher concentration of DGLA in TPPAT as opposed to BPPAT, suggesting a lower activity of delta-5-desaturase in the latter, which corresponds to what has been previously observed in PCa tissue [28]. On the other hand, levels of TPPAT fatty acids reported in this study reveal that there was no decrease in the activity of delta-6-desaturase, contrary to previous observations in PCa tissue [28]. Fatty acid ratios in tissue or plasma for different classes of lipids have been frequently used as an indication of enzyme activity, validating the use of 20:4/20:3 and 20:3/18:2 ratios as indicators of desaturase and elongase activities [24].It is noteworthy that FA content and FA ratios were not statistically significantly different between patients groups for a number of FAs tested. However, this lack of significance seems to be more a function of intragroup variability (as indicated by large SDs) rather than a similarity between PCa and BPH patients. Indeed, the proximity of some of these values to significance suggests that they would probably become significantly different if a larger number of samples were analyzed. Similarly, the lack of correlation between the Gleason score and lipid levels could well be the result of the small number of patients studied. Site-specific differences observed in fatty acid composition of PPAT, both by ourselves and by others [29, 30] suggest that analyzing the fatty acid composition and the lipidome may be important for the understanding of events that occur in the tumor microenvironment. This, in turn, would be helpful to understand which molecules could be involved in the cellular mechanisms that regulate PCa progression and metastasis.
5. Conclusions
- We believe this findings show a possible relationship between the type of lipids/fatty acids present in the periprostatic adipose tissue and the occurrence of prostate cancer. Although it is difficult to determine the type of diet all those patients had in common, mainly because is no usually asked or does not become a significant part of the clinical records, a broader study should take this data into consideration and that would allow to narrow down the molecules possibly involved in cancer progression. Arachidonic acid is a well-known lipid second messenger as well as a metabolite in biologically active molecules (prostaglandins, thromboxanes and leukotrienes, among others) and thus could be a good candidate for further study. Our work did not focus on enzyme activity, approximated only by determining the ratio of substrates and products, and we believe this to be a relevant point in any further investigation, as well as a direct measurement of arachidonic acid metabolites.We believe that the analysis of human periprostatic adipose tissue from Argentine PCa and BPH patients reported on this paper, despite the small number of patients analyzed, contributes significantly to furthering our current knowledge of PCa occurrence and progression, opening the path to further research in the field.Additional studies will be conducted in our laboratory with a larger number of patients in order to correlate the “amount” of fat with the factors produced and / or released by PPAT. We believe that both the study of adipoparacrinology [31] as well as the “mapping” of fat will be a step forward in the study of prostate disease.
ACKNOWLEDGEMENTS
- Valeria Careaga is a CONICET Postdoctoral Fellow. The authors thank the doctors at the Urology Department, Hospital de Clínicas “José de San Martín” for the collection of adipose tissue. The authors want to thank Dr Alejandra Chasseing and Dr Lucrecia Piñeiro for their helpful discussion and assistance with manuscript preparation. This work was supported by a generous donation from Fundación Honorio Bigand and a grant of National Cancer Institute from Argentina (INC).
References
| [1] | Instituto Nacional del Cancer, Ministerio de Salud, Argentina (2012) Available:http://www.msal.gov.ar/inc/index.php/acerca-del-cancer/estadisticas. Last accessed 01/22/2015. |
| [2] | Gross RW, Xianlin H.. Lipidomics at the Interface of Structure and Function in Systems Biology. Chem Biol 2011; 18: 284–1. |
| [3] | Zhou X, Mao J, Ai J, Deng Y, Roth MR, Pound C, Henegar J, Welti R, Bigler SA. Identification of Plasma Lipid Biomarkers for Prostate Cancer by Lipidomics and Bioinformatics. PLOS ONE 2012; 7: e48889. |
| [4] | Lopez Fontana C, Maselli Artola ME, Vanrell Rodriguez MC, Di Milta Monaco NA, Perez Elizalde R, López Laur JD. Advances on the influence of adipose tissue on prostate cancer. Actas Urol Esp 2009; 33: 242-8. |
| [5] | Niclis C, Diaz MP, Eynard AR, Roman MD, La Vecchia C. Dietary habits and prostate cancer prevention: a review of observational studies by focusing on South America. Nutr Cancer 2012; 64: 23-3. |
| [6] | Venkateswaran V, Klotz LH. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat Rev Urol 2010; 7: 442-3. |
| [7] | Schumacher MC, Laven B, Petersson F, Cederholm T, Onelöv E, Ekman P, Brendler C. A comparative study of tissue ω-6 and ω-3 polyunsaturated fatty acids (PUFA) in benign and malignant pathologic stage pT2a radical prostatectomy specimens. Urol Oncol 2013; 31: 318-4. |
| [8] | Matsuyama M, Yoshimura R, Mitsuhashi M, Hase T, Tsuchida K, Takemoto Y, Kawahito Y, Sano H, Nakatani T. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int J Oncol 2004; 24: 821-7. |
| [9] | Kim BH, Kim CI, Chang HS, Choe MS, Jung HR, Kim DY, Park CH. Cyclooxygenase-2 overexpression in chronic inflammation associated with benign prostatic hyperplasia: is it related to apoptosis and angiogenesis of prostate cancer?. Korean J Urol 2011; 52: 253-9. |
| [10] | van Roermund JG, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JL, Kiemeney LA, van Vulpen M. Periprostatic fat correlates with tumor aggressiveness in prostate cancer patients. BJU Int 2001; 107: 1775-9. |
| [11] | Bhindi B, Trottier G, Elharram M, Fernandes KA, Lockwood G, Toi A, Hersey KM, Finelli A, Evans A, van der Kwast TH, Fleshner NE. Measurement of peri-prostatic fat thickness using transrectal ultrasonography (TRUS): a new risk factor for prostate cancer. BJU Int 2012; 110: 980-6. |
| [12] | Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, Zaldivar F, Santos R, Tyson DR, Ornstein DK. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009; 182: 1621-7. |
| [13] | Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Pina F, Mota-Pinto A, Lopes C, Medeiros R. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res 2012; 31: 32-43 |
| [14] | Sacca PA, Creydt VP, Choi H, Mazza ON, Fletcher SJ, Vallone VB, Scorticati C, Chasseing NA, Calvo JC. Human periprostatic adipose tissue: its influence on prostate cancer cells. Cell Physiol Biochem 2012; 30: 113-2. |
| [15] | Ribeiro R, Monteiro C, Catalán V, Hu P, Cunha V, Rodríguez A, Gómez-Ambrosi J, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Pina F, Lopes C, Medeiros R, Frühbeck G. Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. BMC Med 2012; 10: 108-121 |
| [16] | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497-9. |
| [17] | Kelavkar UP, Hutzley J, McHugh K, Allen KG, Parwani A. Prostate tumor growth can be modulated by dietarily targeting the 15-lipoxygenase-1 and cyclooxygenase-2 enzymes. Neoplasia 2009; 11: 692-9. |
| [18] | Suburu J, Chen YQ. Lipids and prostate cancer. Prostaglandins Other Lipid Mediat 2012; 98: 1-10. |
| [19] | Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot.Essent.Fatty Acids 2010; 82: 243-0. |
| [20] | Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 2006; 66: 5977-0. |
| [21] | Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu Rev Biochem 1983; 52: 537-9. |
| [22] | Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J.Cancer 2002; 98: 19-2. |
| [23] | Jones AC, Trujillo KA, Phillips GK, Fleet TM, Murton JK, Severns V, Shah SK, Davis MS, Smith AY, Griffith JK, Fischer EG, Bisoffi M. Early growth response 1 and fatty acid synthase expression is altered in tumor adjacent prostate tissue and indicates field cancerization. Prostate 2012; 72: 1159-0. |
| [24] | Chaudry AA, Wahle KW, McClinton S, Moffat LE. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer 1994; 57: 176-0. |
| [25] | Chaudry A, McClinton S, Moffat LE, Wahle KW. Essential fatty acid distribution in the plasma and tissue phospholipids of patients with benign and malignant prostatic disease. Br J Cancer 1991; 64: 1157-0. |
| [26] | Faas FH, Dang AQ, White J, Schaefer RF, Johnson DE. Decreased prostatic arachidonic acid in human prostatic carcinoma. BJU Int 2003; 92: 551-4. |
| [27] | Sprecher H. Biochemistry of essential fatty acids. Prog Lipid Res 1981; 20: 13-2. |
| [28] | Kelavkar U, Lin Y, Landsittel D, Chandran U, Dhir R. The yin and yang of 15-lipoxygenase-1 and delta-desaturases: dietary omega-6 linoleic acid metabolic pathway in prostate. J Carcinog 2006; 5: 9-14. |
| [29] | Garaulet M, Pérez-Llamas F, Pérez-Ayala M, Martínez P, de Medina FS, Tebar FJ, Zamora S. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 2001; 74: 585-1. |
| [30] | Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cáncer vs hiperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Lekot Essent Fatty Acids 2002; 66: 467-7. |
| [31] | Chaldakov GN, Beltowsky J, Ghenev PI, Fiore M, Panayotov P, Rančič G, Aloe L. Adipoparacrinology--vascular periadventitial adipose tissue (tunica adiposa) as an example. Cell Biol Int 2012; 36: 327-30. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML