-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2014; 3(1): 19-25
doi:10.5923/j.rct.20140301.03
25-Hydroxy Vitamin D: Novel Biomarker for the Diagnosis of Hepatocellular Carcinoma
Eman M. I. Youssef1, Haneya A. A. Ali2, Gehan H. Ewieda1, Wafaa Mohi El-Deen Abd El-Fatah1, Nashwa El-Khouly3, 4
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Egypt
2Department of Microbiology and Immunology, Faculty of Medicine (for girls), Al-Azhar University, Egypt
3Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Internal Medicine, Faculty of Medicine, Taibah University, KSA
Correspondence to: Eman M. I. Youssef, Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
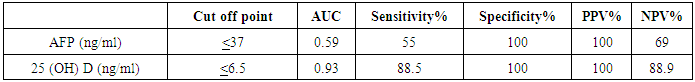
Background: Serum concentration of 25-hydroxy vitamin D (25(OH) D) is the major circulating form of vitamin D and the best indicator of assessment of vitamin D status. It reflects vitamin D obtained from food and supplements and has a fairly long circulating half-life of 15 days. 25(OH)D levels do not indicate the amount of vitamin D stored in body tissues. The non-classical actions of vitamin D which are anti-proliferation, pro-differentiation, pro-apoptosis, anti-inflammation, and immune regulation, have received great attention during the past decade. Vitamin D insufficiency has been associated with the occurrence of various types of cancer, the association between vitamin D status and hepatocellular carcinoma (HCC) has not been well investigated. Objective: This study was designed to evaluate the role of serum vitamin D concentration in the diagnosis of HCC and to consider its potential role as a novel marker for HCC compared with AFP. Subjects and methods: This study was conducted on 80 Egyptian subjects and classified into two groups: HCC group (n = 40), in addition to forty age and sex matched healthy individuals as a control group. HCC was diagnosed histologically or by imaging. Serum 25(OH)D and plasma AFP levels were quantitatively determined using ELISA technique for all participants together with full clinical assessment, liver biochemical profile, viral markers, conventional ultrasound (US) and abdominal triphasic CT scan. Results: Serum 25(OH)D concentration was significantly lower in HCC patients (3ng/ml) than controls (27.25 ng/ml) (P =0.001). Furthermore, the cutoff level of 25(OH) D for diagnosis of HCC in this study was <6.5 ng/ml, with a sensitivity and specificity of 88.5% and 100% respectively. The diagnostic sensitivity of AFP at a cutoff of <37ng/ml was 55% and the specificity was 100%. Conclusions: Serum 25(OH)D could be a potential diagnostic marker for HCC and is relatively comparative to AFP.
Keywords: Hepatocellular carcinoma (HCC), 25-Hydroxy vitamin D(25(OH) D), Alpha-Fetoprotein (AFP)
Cite this paper: Eman M. I. Youssef, Haneya A. A. Ali, Gehan H. Ewieda, Wafaa Mohi El-Deen Abd El-Fatah, Nashwa El-Khouly, 25-Hydroxy Vitamin D: Novel Biomarker for the Diagnosis of Hepatocellular Carcinoma, Research In Cancer and Tumor, Vol. 3 No. 1, 2014, pp. 19-25. doi: 10.5923/j.rct.20140301.03.
Article Outline
1. Introduction
- HCC is a highly aggressive carcinoma of the liver, the fifth most common cancer worldwide, the fourth leading cause of cancer related death [1, 2] and affects more than half a million people annually [3]. In Egypt, chronic hepatitis C virus (HCV) is the main cause of liver cirrhosis and cancer [4]. Risk factors for HCC include; infection with hepatitis B virus (HBV) [5] or HCV, alcoholic and nonalcoholic cirrhosis and exposure to environmental toxins such as aflatoxin [6].HCC is usually diagnosed at late stages, and often has very poor prognosis with limited treatment options [7, 8]. Early diagnosis of HCC is of great importance in order to offer the possibility of curative treatment [9]. AFP and ultrasonography are usually used as diagnostic tools. Serum AFP is the only marker that has been widely used for screening and diagnosis of HCC [10]. However, development of false-negative rates with AFP was as high as 40% for patients with early hepatocellular carcinoma [11]. Thus the identification of novel biochemical markers for HCC remains an important goal around the world [12].Vitamin D is a prohormone with several active metabolites and acts as a hormone. Vitamin D is a fat-soluble vitamin whose principal biological action is to regulate calcium, bone homeostasis [13] and cancer prevention during the last decade due to presence of many physiological processes, including cell growth and differentiation, detoxification of xenobiotic and modulation of adaptive and innate immunity [14, 15] and antineoplastic effects throughanti-proliferative action and programmed cell death [16]. Also, vitamin D can inhibit cancer cell invasion by interfering with specific steps such as angiogenesis and metastasis through decreasing the activity of certain proteases which degrade extracellular matrix and basement membrane [17].HCC is usually asymptomatic in the early stages and tends to be invasive. Therefore, most patients are presented with an incurable disease at the time of detection which makes its early diagnosis critical for a good prognosis [18]. Hence, the aim of this work was to focus on the potential role of serum 25(OH) D as a diagnostic biomarker for HCC and evaluation of its diagnostic utilities in comparison to AFP.
2. Subjects and Methods
2.1. Subjects
- This study was conducted on 40 HCC patientsrecruited from the outpatient clinic of Internal Medicine Department of Al-Zaharaa University Hospital, Cairo, Egypt, from December 2012 till September 2013; in addition to 40 individualswith matched age and sex to the patients were included into the study who served as a control group. All studied individuals included in this study were evaluated by history taking, thorough clinical examination, and laboratory tests including liver functions (alanine aminotransferase (ALT), aspartate aminotransferase (AST), totaland directbilirubin, serum albumin (ALB), international normalized ratio (INR), complete blood picture and viral markers [hepatitis B surface antigen (HBs Ag) and hepatitis C virus antibody (HCV Ab)]. In addition toradiological investigations for diagnosis of HCC was based on one of the following: Ultrasound and computed tomography (CT)] with elevated AFP of more than 400 ng/ml, abdominal triphasic computerized tomography (CT) scan and his to-pathological assessment if needed. Other malignancies or autoimmune diseases were excluded from the study. A verbal informed consent was obtained from all subjects enrolled in the study.Specimens Collection: Seven milliliters of venous blood were obtained from each patient and control subjects with a sterile syringe was drawn without anticoagulant, allowed to stand for 2 hours at room temperature then centrifuged at 3000 rpm for 10 minute. Serum were collected and divided into two aliquots, one for routine laboratory investigations and the other aliquots were stored at -20 C till the time of use, avoid repeated freezing/ thawing.
2.2. Methods
- Measurement of serum AFP by ELISA MethodThe Immunospec AFP is a quantitative solid phase enzyme-linkedimmunosorbent assay (ELISA). AFP was determined using ELISA kit (Catalog No.E1-205) supplied from Immunospec Corporation, 7018 Owens mouth Ave. Suite 103Canoga Park, CA, 91303, according to the manufacturer’s instructions. Detection of Human 25-OH-Vitamin-D by ELISA MethodEnzyme immuno-assay for the quantitative determination of serum 25-OH-vitamin-D concentrations in serum by human enzyme-linked immunoassay 25-OH-Vitamin-D (ELISA) kits (Catalog number: EA300/96, LOTVID1411) [Digital-Life-Design (DLD)], Adlerhorst 15, D-22459 Hamburg, Germany, according to the manufacturer’s instructions, this assay employs the calibrators and patient samples are diluted with biotin-labeled 25-OH Vitamin D and added to microplate wells coated with monoclonal anti-25-OH Vitamin D antibodies. The standard curve from which the 25-OH Vitamin D concentrations in the serum samples can be taken is obtained by point-to-point plotting of the extinction values measured for the 6 calibration sera against the corresponding units (linear/log). Use “4-PL” plotting for calculation of the standard curve by computer.
2.3. Statistical Analysis
- Statistical presentation and analysis of the present study was conducted using Chi-squared test, and that of 2 independent parametric data through Mann Whitney U test by SPSS V 20. The following tests were applied: the X2 test to compare qualitative variables between two independent groups. ROCs were used to evaluate the diagnostic value of 25-OH Vitamin D and AFP and to identify the optimal cut-off values. P-value was considered insignificant at the level of > 0.05, significant at the level of ≤ 0.05 and highly significant at the level of ≤ 0.01.
3. Results
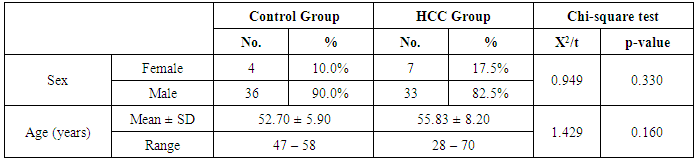
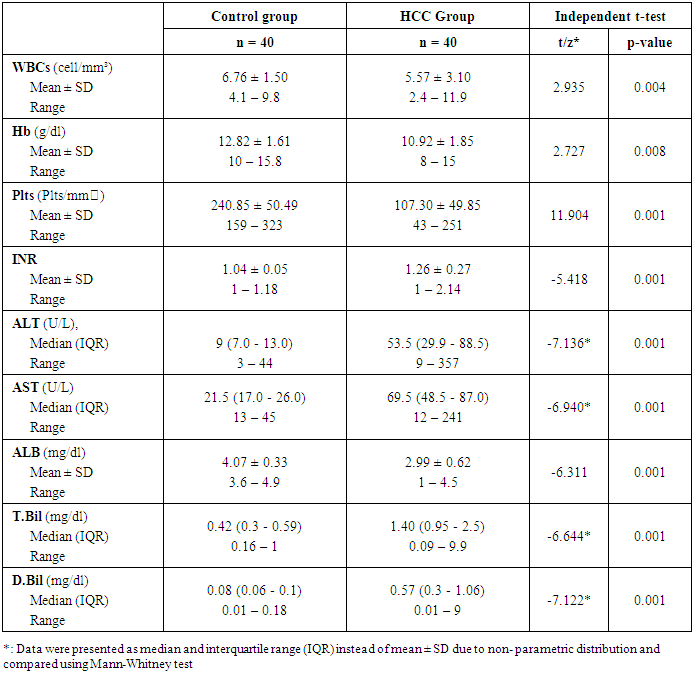
- The results obtained are presented in tables (1-4) and figures (1, 2). HCC group, forty patients (33 males and 7 females) ranged from 28 to 70 years old with a mean age of 55.83 ± 8.20 years and forty healthy control subjects (36 males and 4 females) ranged from 47 to 58 years old with a mean age of 52.70 ± 5.90. Characteristics of the patients with HCC and healthy controls are presented in table (1). Detailed laboratory data of all studied groups are shown in table (2).
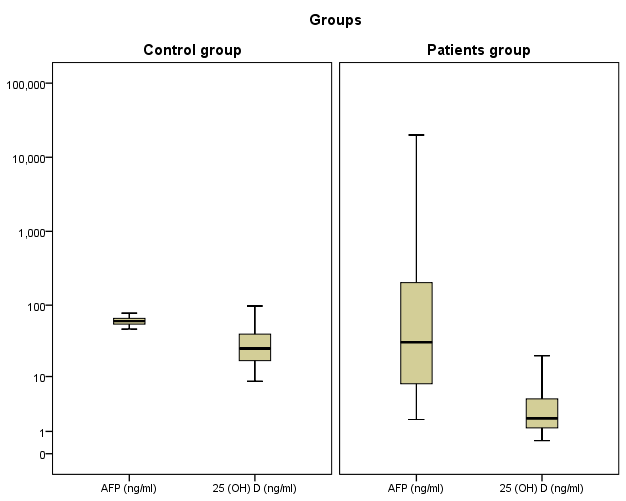 | Figure 1. Median of AFP and 25 (OH) D in two studied groups |
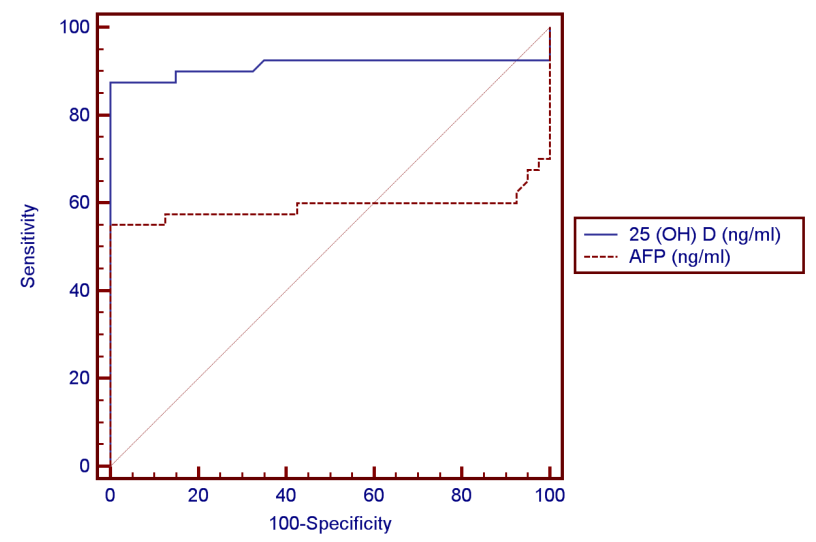 | Figure 2. ROC curve for AFP versus 25 (OH) D in diagnosis of HCC |
|
|
|
|
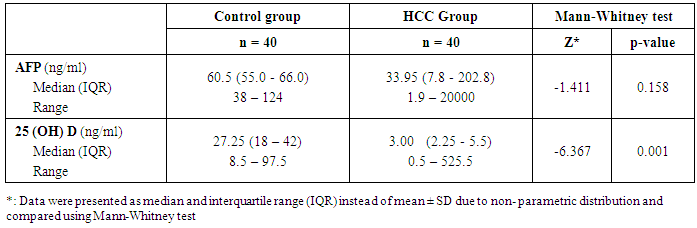
- Regarding the results of median serum levels of vitamin D concentration was significantly higher in healthy controls compared to HCC patients (P ≤ 0.001). On the other hand, there is no significant difference between both groups as regard AFP. Correlation of 25(OH) D concentration was made with different laboratory parameters, it was found a significant negative correlation between it and ALT, AST (-0.680, P≤0.001) and (r = - 0.605, P ≤ 0.001) respectively) Also, a negative correlation between it and AFP was found but not significant (r = - 0.053, P = 0.843).In receiver operator characteristic (ROC) curve analyses to study the HCC diagnostic ability of both markers, 25 (OH) D (ng/ml) at the best cut-off value (< 6.5ng/ml) had sensitivity of 88.5%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 88.9% for detection of HCC patients (are aunder the curve (AUC) = 0.93). The data of ROC analysis for AFB at a cut-off value of <37 ng/ml had sensitivity of 55% and specificity of 100% (Table 4 and Figure 2).
4. Discussion
- A number of studies investigated the relations between serum vitamin D levels and various cancers such as cancer of the colorectal [19], esophagus [20], lung and ovaries [21], prostate [22] and pancreas [23], little epidemiologic data for vitamin D and liver cancer are available, despite the important role of the liver in metabolizing the circulating form of vitamin D [24]. HCC is now a rather common malignancy in Egypt which usually develops on top of liver cirrhosis secondary to viral infection [25]. It is three times more common in men than women, which could be explained by differences in exposure to risk factors [26].The results of the present study were higher than in a study done in Egypt by Hammad et al. [27], who studied series of 1328 with HCC cases and they reported that HCC is significantly higher in men than women (77.7 and 22.3%, respectively). The present study demonstrated that 25 (OH) D levels significantly differed between two groups, where median serum 25 (OH) D levels was significantly lower in HCC (3ng/ml) than healthy controls (27.25 ng/ml) (P ≤0.001). On the contrary, there is no significant difference between both groups as regard AFP. These results were similar to a recent prospective cohort study done by Finkelmeier et al. [28]. 25-hydroxyvitamin D3 (25(OH)D3) levels were subsequently measured in two-hundred patients with HCC. 25(OH)D3 levels were compared to stages of cirrhosis and HCC. The mean serum 25(OH)D3 concentration was 17 ± 13 ng/mL with a range of 1-72 ng/mL. 25(OH)D3 serum levels negatively correlated with the stage of cirrhosis as well as with stages of HCC. Patients with severe 25(OH)D3 deficiency had the highest mortality risk and high AFP levels (>400 ng/mL). They concluded that 25(OH)D3 deficiency was associated with advanced stages of HCC and it is a prognostic indicator for a poor outcome. Also, our results were in agreement with a study done by Hammad et al. [27], who found a significant progressive decline in the active vitamin D status was noted in all three clinical states (patients with peri-hepatic fibrosis, hepatic cirrhosis, or HCC) and these too were associated with progressive liver disease.Similarly, Lange et al. [26], who measured the largest study of non-cirrhotic patients with chronic HCV infection. Vitamin D status was assessed in a cohort of 468 patients. The average 25(OH)D level was 17 ng/ml and 25% of the patients had levels below 10 ng/ml. They concluded that the prevalence of vitamin D deficiency was greater in patients with more advanced fibrosis. Also they provided evidence for a functionally relevant contribution of reduced 25(OH)D serum levels to the risk of developing hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC). Additionally, Mansoor et al. [29], they made a study of prevalence and significance of vitamin D deficiency and insufficiency among apparently healthy adults found high prevalence of 25(OH) vitamin D deficiency 90% had low serum 25(OH)D levels (69.9% were deficient and 21.1% had insufficient levels of 25(OH)D among apparently healthy adults, hospital staff and health care professionals. And also, Fisher et al. [30] evaluated vitamin D levels in 100 patients with liver disease, 51 with cirrhosis and 49 without cirrhosis, including 38 patients with chronic hepatitis C. They concluded that the prevalence of vitamin D deficiency was significantly higher in cirrhotic than non-cirrhotic subjects (86.3% versus 49.0%, p = 0.0001). Vitamin D insufficiency has been previously linked to the development of HCC [31]. However, causal relationships remained mostly unclear because the most studies were small or concentrated on the assessment of 25(OH)D3 serum levels at the date of HCC, which may result in false-positive associations due the influence of impaired liver function on circulating 25(OH)D3 [32]. Another study done by Caputo et al. [33], they found that the inhibitory effect of 1,25-dihydroxyvitamin D3 on growth of the human liver cancer cell line which express functional receptors able to specifically bind 1,25-(OH)(2)D3. The highest level of functional receptor was found in the human liver cancer cell line resulted from arrest in the G0/G1 phase of the cell cycle. For the diagnosis of HCC in the current study, ROC curve was made to detect a cutoff value for diagnosis of vitamin D deficiency that was found to be equal or less than 6.5ng/ml. Serum levels of 25-hydroxyvitamin D showed 88.5% sensitivity, 100% specificity, 100% PPV and 88.9% NPV and AUC 93%. In addition to, sensitivity and specificity of AFP were 55% and 100%, respectively, at a cutoff value ≤ 37 ng/ml. At this level, the AUC, PPV and NPV were 59%, 100% and 69% respectively, which suggests that determining 25 (OH) D levels might be superior to AFP levels in diagnosis of HCC. These findings from our study in accordance with previous study made by Falleti et al. [34], found vitamin D deficiency in large proportion (55.8%) in their patients with HCC where vitamin D deficiency was diagnosed according to a cutoff value < 15ng/ml. They concluded that the vitamin D receptor (VDR) genetic polymorphisms are significantly associated with the occurrence of HCC inpatients with liver cirrhosis. This relationship is more specific for patients with an alcoholic etiology. The most common used laboratory marker for diagnosis is alpha-fetoprotein. However, it has a high rate of false-negative and false positive results, as revealed by Samir et al. [35], who stated that sensitivity and specificity depend on the cut-off value chosen. In HCC-cirrhotic patients, using a cut-off level of 20ng/mL, sensitivity is only around 60% and specificity ranges from 80% to 94%. This prompted the need of other reliable markers for this disease. Also, Giannini et al. [11], who concluded that serum AFP levels have no prognostic meaning in well-compensated cirrhosis patients with single, small HCC.
5. Conclusions
- In this study, it was concluded that the serum level of 25(OH) D is significantly lower in HCC patients in comparison to controls. A receiver operating characteristics (ROC) analysis suggests that serum 25 (OH) D could be a biomarker for HCC.It is recommended to carry out further studies with a larger sample size are mandatory to underline the accuracy of our findings before their application at the population level. Additional studies are necessary to gain greater insight into the impact of 25(OH) D in the pathogenesis of HCC and its potential usefulness in HCC patients. Further combining 25(OH) D with gene expression or with other diagnostic markers might increase the accuracy of HCC diagnosis. Studying of 25(OH) D in different grades and various stages of HCC with follow up of patients over several years will reveal the role of 25(OH) D in predicting prognosis.Further clinical investigations on the effect of vitamin D supplementation in treating CHC are needed. Supplementation with daily vitamin D is recommended to avoid hazards of vitamin D deficiency.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML