-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2014; 3(1): 6-18
doi:10.5923/j.rct.20140301.02
Angiopoietin-2/Tie2 Signaling in the Microenvironment of Chronic Lymphocytic Leukemia (CLL)
Birgit Pötzsch1, Iris Gehrke2, Simon Poll-Wolbeck1, Hanna Flamme1, Karl-Anton Kreuzer1, 3
1Department I of Internal Medicine, University at Cologne, Cologne, Germany
2Manitoba Institute of Cell Biology, University of Manitoba, Winnipeg, Canada
3Department I of Internal Medicine, University at Cologne Kerpener Strasse 62 50937 Cologne, Germany
Correspondence to: Karl-Anton Kreuzer, Department I of Internal Medicine, University at Cologne, Cologne, Germany.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. It is characterized by an accumulation of B-lymphocytes in peripheral blood (PB), bone marrow (BM), lymph nodes (LN) and spleen. Tissue involvement is strongly associated with disease progression and aggressiveness. Angiogenesis is the process of blood vessel generation out of existing ones. Increased angiogenesis in BM of CLL-patients has been associated with advanced disease stage and aggressiveness. Also high levels of the angiogenic factor angiopoietin 2 (Ang2) have been demonstrated to be present in plasma of CLL-patients and to correlate with adverse prognosis. While available data about Ang2 in CLL is solely descriptive, no information is available about the presence and possible functional relevance of the only known Ang2-receptor Tie2 in CLL. This study aimed on a systematic analysis of the Ang2- and Tie2-status of CLL-cells within their different microenvironments as well as cellular components as possible interaction partners through the Ang2/Tie2 axis within these microenvironments. We confirmed expression and secretion of Ang2 in CLL-cells, while Tie2 was absent in PB-derived CLL-cells. CD40L/IL4-stimulation and co-culture with feeder cell lines HS5, NKtert, KUSA or HUVEC improved survival of isolated CLL-cells as expected. At the same time, neither Ang2-secretion, nor Tie2-expression was altered in CLL-cells upon these stimuli. Hypoxia significantly increased Ang2-secretion of CLL-cells, but not Tie2-expression. We detected Tie2 on granulocytes of CLL-patients as well as on healthy volunteers. Interestingly, we identified bone marrow-derived CLL-cells to express Tie2 as well as primary BM-stroma cells. Co-culture with in vitro-differentiated nurse-like cells (NLCs) served as a mimic of the lymph node microenvironment. Neither CLL-cells co-cultured with NLCs, nor NLCs themselves expressed Tie2. Last, we could show that neither co-culture mediated survival advantage, nor survival of monocultured CLL-cells was altered upon blocking of Ang2 by the peptibody AMG386. We conclude, Ang2 may act in an autocrine fashion in BM-associated CLL-cells. Also BM-stroma cells and endothelial cells, due to their positive Tie2-status, represent potential cell types for receiving Ang2 signals in BM. Based on their Tie2-positivity, granulocytes may be potential interaction partners for CLL-cell derived Ang2 in PB. As hypoxia does not lead to increased Tie2-expression in PB-derived CLL-cells, Ang2/Tie2 induction and action may be altered from classical angiogenesis-related signaling. These results considerably contribute to understanding the CLL-cell-microenvironment interaction via the Ang2/Tie2 axis.
Keywords: Chronic lymphocytic leukemia, Angiogenesis, Tie2, Ang2, CLL-microenvironment
Cite this paper: Birgit Pötzsch, Iris Gehrke, Simon Poll-Wolbeck, Hanna Flamme, Karl-Anton Kreuzer, Angiopoietin-2/Tie2 Signaling in the Microenvironment of Chronic Lymphocytic Leukemia (CLL), Research In Cancer and Tumor, Vol. 3 No. 1, 2014, pp. 6-18. doi: 10.5923/j.rct.20140301.02.
Article Outline
1. Introduction
- Chronic lymphocytic leukemia (CLL) is a frequent leukemia in adults and remains up to date incurable with standard treatment options. The disease is characterized by an accumulation of immune-incompetent, monoclonal B-lymphocytes with mature phenotype. The etiology remains not fully understood. While CLL-cells feature an apoptotic defect which contributes to their accumulation in vivo, they die rapidly after being isolated when cultured in vitro. Hence, the microenvironment has been assigned a critical role in CLL-pathophysiology [1–3]. While not only Rai-stage classification of CLL is based on increased tissue-involvement in advanced disease stage, evidence suggests, that especially tissue-associated leukemic cells are the disease-driving population largely accounting for disease aggressiveness, drug resistance and relapse [2, 3]. The microenvironment of the CLL-cell includes several cell types. Amongst others, peripheral blood cells (T-cells, monocytes, granulocytes), bone marrow stromal cells (BMSCs) [4–6] or nurse like cells (NLCs) [7], primarily present in proliferation centers in the lymph node. These heterogeneous cell populations create dynamic microenvironments involving direct cell-cell interactions with high concentrations of growth factors and cytokines present. Several secreted factors (e.g. CD40L, IL4), as well as coculture with primary cells (e.g. BMSCs sand NLCs) or cell line (e.g. HS5, KUSA, NKtert) feeder cells have been described to improve survival in culture by partially mimicking the in vivo microenvironment, thereby allowing studying CLL-cell-microenvironment interactions particularly relevant aggressive CLL, drug resistance and relapse [5, 8, 9].Angiogenesis is the process of vessel formation out of preexisting ones. Under physiological conditions angiogenesis is induced by hypoxic conditions and plays a role in processes such as wound healing and pregnancy [10; 11]. Under patho-physological conditions the highly controlled balance between pro- and anti-angiogenic factors is disturbed towards higher levels of pro-angiogenic factors, such as vascular endothelial growth factors (VEGF) and Angiopoietin 2 (Ang2) [12]. In solid tumor biology these increased levels of angiogenesis are critical for supplying the growing tumor mass with sufficient oxygen and nutrients [13]. Due to absence of an actual solid tumor mass, the role of angiogenesis and angiogenic factors is rather underexplored in hematologic malignancies. The bone marrow of CLL-patients has been described to possess increased microvessel density compared to leukemic, age-matched donors [14]. Also, VEGF has been demonstrated to be over-expressed and secreted by CLL-cells, and plasma/serum VEGF has been correlated with adverse prognostic markers, disease progression and overall survival (15; 16). VEGF has also been assigned an angiogenesis-independent role in CLL-cell survival as well as relevance in CLL-cell/microenvironment interactions [15]. Less studied in this regard is the pro-angiogenic factor Ang2. Ang2 acts via binding to its receptor Tie2. This interaction disrupts the binding of Ang1 to Tie2, thereby interfering with the quiescent phenotype of the resting endothelium which is maintained by the Ang1/Tie2 interaction [11, 17]. Ang2 has been detected in plasma / serum of CLL-patients and associated with adverse prognostic factors and advanced disease stages. Also, variable Ang2 expression by CLL-cells [14, 16, 18–20] as well as Ang2 secretion of cultured CLL-cells was described [14, 21].While up to date no data about the Ang2-receptor Tie2 is available for CLL, Tie2 was found on acute myeloid leukemia (AML) cells and the Ang2/Tie2 system was suggested to be involved in leukemogenesis and chemosensitivity in human AML [22, 23]. Also a bidirectional crosstalk between AML-cells and Tie2-expressing endothelial cells, as well as an angiogenesis-independent role in proliferation and survival of malignant AML-cells has been suggested [24].We hypothesize, that the Ang2/Tie2 axis may present a route of bidirectional interactions between CLL-cells and their microenvironment in the bone marrow and/or the lymph node. The Ang2/Tie2 axis may thereby contribute to the phenotype of tissue-associated CLL-cells, which are believed to represent the most aggressive CLL-cell population accounting for disease progression and relapse. The results may suggest new possibilities for therapeutically targeting the CLL-cell microenvironment interactions.
2. Materials and Methods
- Patients: Peripheral blood (PB) and bone marrow (BM) aspirates from consented CLL-patients with a confirmed diagnosis according to standard criteria [25] were utilized. CLL-patients were either untreated or off treatment for at least three months prior to blood or bone marrow withdrawal. The study was performed according to the World Medical Association's Declaration of Helsinki (6th version, Seoul, South Korea, 2008) and authorized by the local ethics committee (approval number 04-231). Primary cells and plasma: Whole blood was centrifuged for 15 min at 1,600 rpm. Plasma was extracted and stored at -80°C until further use. PBMCs were isolated from PB of CLL-patients or age-matched healthy volunteers by Ficoll Hypaque density gradient centrifugation. CLL-cells were enriched by RosetteSep™ (Stem Cell Technology, Colone, Germany) incubation prior to density gradient centrifugation. Freshly isolated cells were placed into RPMI1640 culture medium (Biochrom AG, Berlin, Germany) containing 1% penicillin/streptomycin (P/S; Biochrom AG) and 10% fetal bovine serum (FBS; Biochrom AG) at 37°C and 5% CO2 in a humidified atmosphere.BM-derived CLL-cells: Mononuclear cells from BM aspirates from CLL-patients were extracted by Ficoll Hypaque density gradient centrifugation and utilized for mRNA extraction to perform PCR. For flow cytometric analyses of Tie2 surface expression BM aspirates were freed from erythrocytes by incubation with hypotonic solution (RBC lyis buffer, Qiagen, Hilden Germany) and subject to antibody incubation.Generation of BM-stromal cells (BMSCs): For generation of BMSCs, Ficoll Hypaque-isolated mononuclear cells were plated at 1x10*7/ml in RPMI1640 (Biochrom AG)/ 1% P/S (Biochrom AG), 10% FBS (Biochrom AG) and incubated at 37°C and 5% CO2 in a humidified atmosphere. After two days non-adherent cells were removed and fresh medium (10% of total volume) was added every three days thereafter. After three weeks adherent primary human BM-stromal cells were detached by 2mM EDTA solution and subject to mRNA isolation for PCR.Generation of nurse like cells (NLCs): For generation of NLCs, PBMCs from CLL-patients were cultured at 1x10*7/ml in RPMI1640, 1% P/S, 10% FBS for three weeks at 37°C and 5% CO2 in a humidified atmosphere. Fresh medium (10% of total volume) was added every three days. NLCs develop from monocytesin the presence of CLL-cells and represent a heterogeneous population of large round and fibroblast-like adherent cells. Non-adherentCLL-cells were removed and NLCs were detached by 2mM EDTA solution. NLCs and CLL-cells were subject to flow cytometric assessment of Tie2-status. HUVECs, cell lines and coculture: HUVEC, isolated from normal human umbilical vein, were purchased from PromoCell GmbH (Heidelberg, Germany) and cultured in Endothelial Growth ready-to-use Medium including growth factor supplement (PromoCell). HUVECs were detached by 2 mM EDTA and used for experiments between passages three and eight. The human BM stromal cell lines HS5 and NKtert, as well as the mouse BM stromal cell line KUSA were utilized for co-culture experiments. Cell lines were cultured in RPMI1640/1% P/S, 10% FBS at 37°C and 5% CO2 in a humidified atmosphere and split accordingly their growth kinetics. For coculture experiments, stromal cell lines were seeded at 4x10*5/ml and allowed to attach over night. Primary CLL-cells were added at 4x10*6/ml.Cell treatments: Primary CLL-cells were incubated in RPMI1640 culture medium (Biochrom AG) containing 1% penicillin/streptomycin (P/S; Biochrom AG), 10% fetal bovine serum (FBS; Biochrom AG) supplemented with 10 µg/ml CD40L, 5 ng/ml IL4, a combination of both at 37°C and 5% CO2 in a humidified atmosphere for 24h. Thereafter, survival was assessed by Annexin V/PI staining, supernatant was collected for Ang2-content by ELISA and Tie2 status was assessed flow cytometrically as described. Further, primary CLL-cells or HS5 cells were cultured in normoxia (21% O2) in standard incubator (5% CO2, humidified atmosphere) or under hypoxic conditions in a modified incubator (2% O2, 5% CO2, humidified atmosphere). Standard RPMI medium was equilibrated in hypoxic conditions for 2days prior to be used for cell culture. Supernatant was collected after 24h in normoxia or hypoxia for ELISA-based assessment of secreted Ang2. For flow cytometric analysis of Tie2 cells were washed once, incubated with anti-Tie2 PE antibody and measured and analysed as described. For anti-Ang studies HUVEC or HS5 cells were seeded at 4x10*5/ml in RPMI1640 and allowed to adhere overnight. CLL-cells were seeded onto adherent HUVEC or HS5 feeder layer or monocultured at 4x10*6/ml. AMG386 (Trebananib) was added at 10µg/ml [26]. Cells were harvested after 24h and viability was assessed by flow cytometrically by Annexin V-FITC/PI staining. Polymerase chain reaction (PCR): Primary cells were subject to mRNA isolation (RNeasy Plus Mini Kit, Qiagen, Hilden Germany) followed by reverse transcription into cDNA (Revert aid premium reverse Transkriptase, Fermentas, St. Leon-Rot, Germany).The following primers (Tib MOLBIOL, Berlin, Germany) were used: Tie2 forward: 5’ CGAGTTCGAGGAGAGGCAAT, Tie2 reverse: 5’ TCAGGTACTTCATGCCGG, Ang2 forward: 5’GACGCGCCGCTCGAATACGA and Ang2 reverse: 5’TCCGCGTTTGCTCCGCTGTT. ABL was used as house keeping control: forward:5´TGGAGATAACACTCTAAGCATAACTAAAGGT, reverse:5`GATGATGTTGCTTGGGACCCA. The Fast Start PCR Master Kit (Roche, Mannheim, Germany) was used for PCR set up. Denaturation was carried out at 95°C and elongation at 72°C for all primers. Annealing temperature for Tie2 was 55°C, 65°C for Ang2, and 60°C for ABL. All PCRs ran for 40 cycles. PCR product was separated and visualized by QIAcxel capillary electrophoresis (Qiagen).DNA Sequencing: Sequencing was carried out with the Big Dye Terminator v 1.1 Cycle Sequencing Kit (Life technologies,Applied Biosystems, Darmstadt, Germany) following the method of Sanger. The following primers (Tib MOLBIOL) were used: Set1 forward:5’GTAGCAGCCCTGCGTTTTAG, reverse:5’CCATTTCTTTCTTCATGTTGTCC, Set2 forward: 5’GCAAGTGCTGGAGAACATCA, reverse: 5’TGATGTGCTTGTCTTCCATAGC, Set3 forward: 5’GATTTTGGACCAGACCAGTGA, reverse: 5’AGTTTGATGTGGACATCATAGTCA3’, Set4 forward: isoform 1 5’CTATGATGTCCACATCAAACTCAG, forward: isoform 25’ACTATGATGTCCACATCAAACTCTA, reverse: 5’TTGGCTGATGCTGCTTATTTT, Set5 forward: 5’TGAGGCTTACTCATTGTATGAACA, reverse: 5’GAGACAGTTCCTCAGGTGGAC. Touchdown PCR was carried out for every primer setstarting at 60°C for annealing with 0.5°C temperature reduction for 10 cycles, followed by 30 cycles at 55°C annealing temperature. Sequencing was carried outby Sanger method using capillary electrophoresis on the Applied Biosystems 3730 DNA analyzer.ELISA: ELISA was carried out using the Quantikine Human Ang2 Detection Kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer´s instructions. Primary CLL-cells or healthy PBMCs were cultured at 4x10*6/ml, while cell lines were cultured at 4x10*5/ml. After 24 h supernatant was freed from suspension cells by centrifugation (6000 RPM, 2min) and stored at -80°C until use. Plasma samples were diluted 1:5 with diluent buffer provided by the kit prior to incubation. Each sample was tested in duplicate. The concentrations are reported in pg/ml.Flow cytometry: For analysis of Tie2-status in primary CLL B-cells as well as B-cells from healthy donors whole blood was utilized. Bloodwas washed and incubated with CD5 FITC antibody, CD19 PerCPCy5.5 antibody (both BioLegend, San Diego, California, USA) and anti-Tie2 PE antibody (R&D Systems, Minneapolis, MN, USA) or PE-labelled isotype control (BioLegend), followed by erythrocyte lysis through incubation with hypotonic buffer. Lymphocytes were selected based on distribution in side/forward scatter. Tie2-status was assessed on CD5/CD19 double positive CLL-cells or CD5 negative/CD19 positive B-cells from healthy donors. Tie2-status in HUVECs and isolatedprimary cells as well as BMSCs and NLCs was addressed by detaching cells by EDTA incubation (BMSCs and NLCs only), washing cells, followed by incubation with anti-Tie PE antibody or PE-labelled isotype control. Fluorescence was measured and analysed with FACSCanto, FACSDiva 3.0 software (BD Bioscience Heidelberg, Germany) and Cyflogic 1.2.1 software.Viability of CLL-cells was assessed by the Annexin V-FITC/PI apoptosis detection kit I following the manufacturer´s instructions (BD Bioscience Pharmingen, Heidelberg, Germany). Fluorescence was measured and analysed with FACSCanto and FACSDiva software (BD Bioscience). Annexin V binds phosphatidylserine, which is only accessible on the cell surface upon apoptosis induction. Annexin V-positive, but PI-negative cells are therefore considered early apoptotic, while Annexin V/PI double positive cells are considered late apoptotic or dead. Survival was assessed after 24h in culture, with or without treatment/supplement or coculture.
3. Results
3.1. Ang2-status in Leukemic Cells from CLL-patients and Healthy Donors
- Plasma Ang2 content was assessed by ELISA in CLL-patients and healthy donors. Ang2-levels were significantly higher in plasma from CLL-patients (n=9, 2529pg +/- 253pg/ml) compared to Ang2-levels in plasma of healthy donors (n=4, 1267pg +/- 78pg/ml) (Fig. 1A). Further,the expression-status of Ang2 in CLL-cells and healthy volunteers was assessed by PCR. The nine analysed CLLsamples show various degrees of Ang2-expression ranging from no expression to expression comparable to the strongly positive control HUVEC (pos ctrl). Healthy PBMCs showed only very weak (samples 1,3 and 4) to no (sample 2) Ang2- expression (Fig. 1B). To address the question whether CLL-cells themselves contribute to plasma Ang2 by protein secretion, isolated CLL-cells or healthy PBMCs were cultured for 24h and secreted Ang2 in supernatant was measured by ELISA. CLL-cells secreted significantly higher levels of Ang2 compared to healthy PBMCs with 116 pg +/- 24 pg/ml and 26 pg +/- 2 pg/ml, respectively (Fig. 1C). To further characterize Ang2 in CLL, the Ang2-gene was sequenced. Out of three assessed patient samples, one patient sample featured two mutations, one featured four mutations and the third patient sample did not show any mutations. HUVECs did not feature any mutations in the Ang2 gene. The mutations found in CLL-patients were point mutation and did not lead to an exchange in amino acids. All in this study identified point mutations have been described for Ang2 before. Results are summarized in table 1.
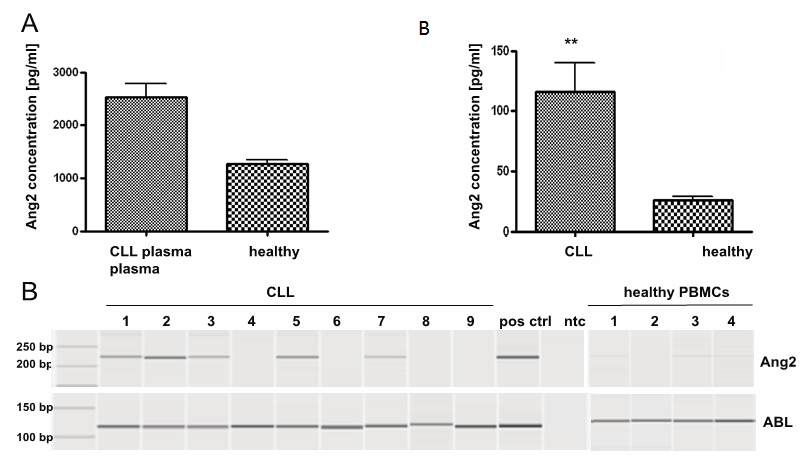 | Figure 1. Ang2 status in primary CLL-cells and PBMCs from healthy donors: (A) Plasma of CLL-patients or healthy donors was analysed for Ang2 content by ELISA. Ang2-levels were significantly higher in CLL-plasma compared to plasma from healthy donors (p<0.01). (B) mRNA was isolated from CLL-cells or PBMCs from healthy donors and converted into cDNA. Ang2 specific primers were used to determine Ang2-mRNA levels by PCR. ABL-specific primers served as housekeeping control. HUVEC functioned as positive control (pos ctrl) for Ang2. A non template control (ntc) served as contamination control. PCR product was analysed by capillary gel electrophoresis. Ang2 was heterogeneous in CLL cells and largely absent in healthy PBMCs. (C) Isolated CLL-cells or healthy PBMCs were cultured under standard conditions and Ang2 levels in supernatant were assessed after 24h by ELISA. Ang2-levels were significantly higher in supernatant of CLL-cells compared to supernatant from healthy PBMCs (p<0.01). Significances were calculated using Mann-Whitney U-test |
3.2. Tie2 Status in Leukemic Cells from CLL-patients and Healthy Donors
- The Tie2-status of CLL-cells has not been described up to date. We assessed the expression and the protein-status of Tie2 in CLL-cells by PCR and flow cytometry. We did not see Tie2-expression in any of the tested CLL samples (n=9), while low expression of Tie2 was observed in PBMCs from healthy donors (Fig. 2A). As PBMCs are not a pure B-cell population this may be due to contribution of Tie2-positive monocytes which have been described to account for about 2 to 7% of monocytes in PBMCs from healthy donors [27]. This assumption could be proved by flow cytometry, where healthy B-cells where negative for Tie2 demonstrated by a representative histogram (Fig.2B, middle). Negative Tie2-expression in CLL-cells was confirmed on the protein level by flow cytometry as shown by a representative histogram (Fig.2B, left). A summary of MFIs for Tie2 of CLL-cells (n=4), healthy B-cells (n=5) and HUVEC, functioning as positive control, are depicted in Figure 2C.
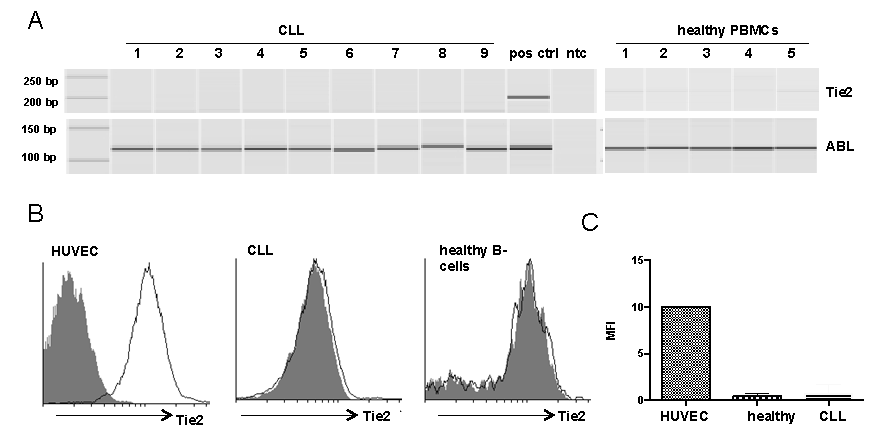 | Figure 2. Tie2 status in primary CLL-cells and PBMCs from healthy donors(A) mRNA was isolated from CLL-cells or PBMCs from healthy donors and converted into cDNA. Tie2 specific primers were used to determine Tie2-mRNA levels by PCR. ABL-specific primers served as housekeeping control. HUVEC functioned as positive control (pos ctrl) for Tie2. A non template control (ntc) served as contamination control. PCR product was analysed by capillary gel electrophoresis. Tie2 was absent in CLL-cells and weakly expressed in healthy PBMCs. (B) CLL or healthy donor whole blood was stained with CD5-FITC, CD19-PerCPCy5.5 and Tie2-PE. Tie2 expression was analysed on CD5/CD19-double positive cells in blood from CLL-patients and in CD19-positive/CD5-negative B-cells from healthy donors. HUVEC functioned as positive control for Tie2. Representative histograms visualize absence of Tie2 on CLL-cells and healthy B-cells. (C) Median fluorescent intensity (MFI) values for Tie2 are summarized for all samples tested |
3.3. Ang2 and Tie2 Status in CLL-cells upon Microenvironmental Stimulation
3.3.1. CD40L and IL4
- To address the question whether microenvironmental stimuli impact on the Ang2- and/or Tie2-status in primary CLL-cells, CLL-cells were stimulated with the physiological stimuli IL4 and CD40L. Both stimuli are known to prolong in vitro survival of isolated CLL-cells [8, 9, 28, 29], thereby restoring parts of their in vivo physiology. In our experiments CD40L and IL4 improved in vitro survival after 24h in culture by about 10% for either stimuli alone and about 18% for a combination of both (n=4, Fig. 3A).At the same time, neither IL4 (n=4), nor CD40L (n=6), nor a combination of both (n=4) had a significant impact on Ang2 secretion (Fig. 3B) or Tie2-surface protein levels (n=4, Fig. 3C) in CLL-cells as assessed by ELISA and FACS, respectively. Figure 3C shows a representative histogram (right) and a bar chart summarizing results obtained for four samples assessed (left).
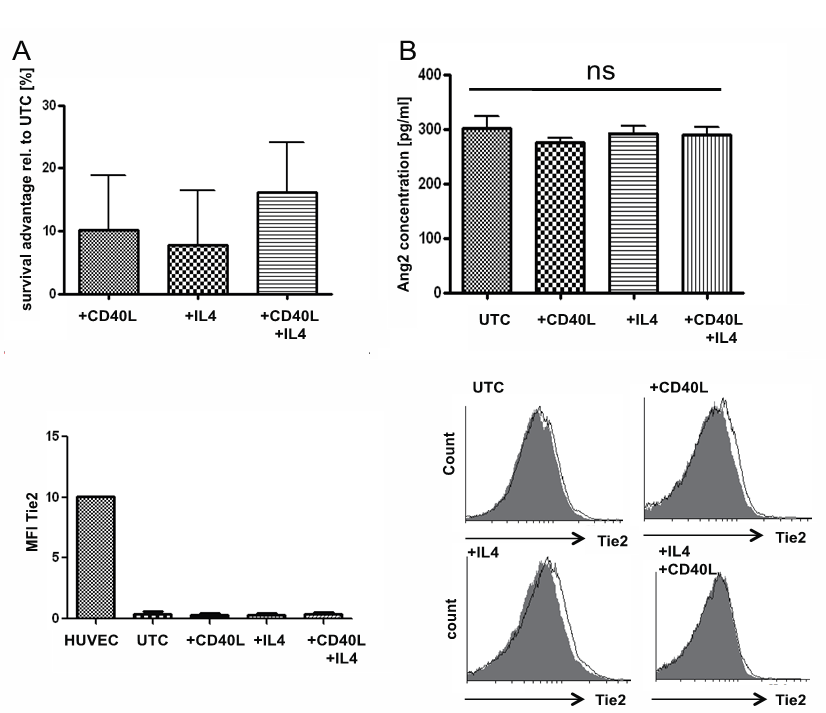 | Figure 3. Ang2 and Tie2 status upon CD40L and IL4 stimulation: Primary CLL-cells were incubated in absence (untreated control UTC) or presence of CD40L at 10µg/ml, IL4 at 5ng/ml or a combination of both for 24h. (A) Cell viability was assessed by flow cytometric measure of Annexin V-FITC/PI. Annexin V/PI-double negative cells were considered viable. Stimuli conferred a survival advantage of around 10% with single stimulus and around 18% when both stimuli were combined. (B) Ang2-concentration in supernatant was assessed by ELISA. No significant (ns) effect of either stimulus alone or in combination on Ang2 secretion could be detected. (C) Tie2 surface expression was assessed by flow cytometry after 24h of culture with or without CD40L, IL4 or a combination of both using a PE-labeled Tie2 antibody. No significant (ns) effect of either stimulus alone or in combination on Tie2 surface levels could be detected. Median fluorescent intensity (MFI) values for Tie2 are summarized for all samples tested (left). One representative histogram for each setting is depicted on the right. Significances were calculated using Mann-Whitney U-test |
3.3.2. Co-culture with Feeder Cell Lines
- For a more complex mimic of the in vivo situation, CLL-cells were co-cultured with several feeder cell lines. These feeder cells included the human bone marrow stromal cell line HS5, the mouse bone marrow stromal cell lines NKtert and KUSA and the human endothelial cell line HUVEC. All feedercell lines improved in vitro survival of CLL-cells compared to monocultured CLL-cells(n=6Fig. 4A). KUSA, NKtert and HS5 cell lines secreted Ang2 at levels comparable to those secreted by CLL-cells themselves, while HUVEC, as expected, secreted about 5 fold more Ang2 after 24h in culture (Fig. 4B). Ang2-levels in supernatant of co-cultures did not significantly differ from monocultures of either cell type (n=9, Fig. 4B). Interestingly, there was no additive effect seen in co-cultures, suggesting an interaction between CLL-cells and feeder cells which negatively impacts on Ang2-production and/or secretion. Further, co-cultures did not increase Tie2-levels on CLL-cells as demonstrated by flow cytometry of surface Tie2 (Co HUVEC n=2, Co NKtert n=6, Co KUSA n=5, Co HS5 n=6, Fig. 4C). The bar chart demonstrates average MFIs for all assessed samples (Fig. 4C top), while the histograms depict results from one representative sample for each setting (Fig. 4C bottom).
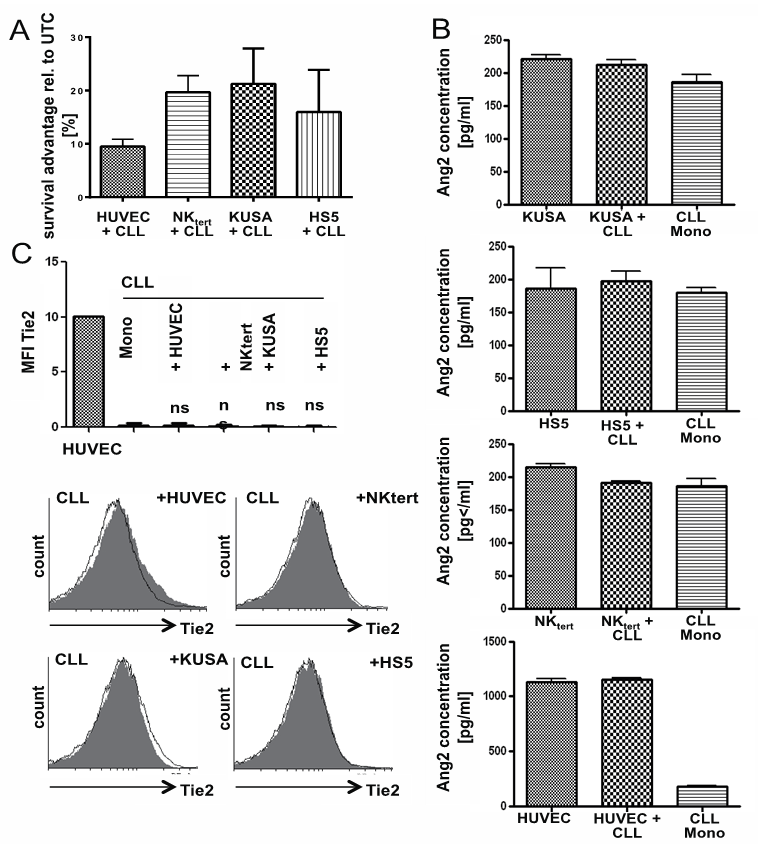 | Figure 4. Ang2 and Tie2 status upon co-culture with feeder cell lines: Primary CLL-cells were incubated in absence (untreated control UTC) or presence of the feeder cell lines HUVEC, NKtert, KUSA or HS5. (A) Cell viability was assessed by flow cytometric measure of Annexin V-FITC/PI. Annexin V/PI-double negative cells were considered viable. Co-cultures resulted in a survival advantage of around 13,84% for HUVEC, 15,68% for NKtert, 21,25% for KUSA and 15,39% for HS5 co-culture. (B) Ang2 levels were determined by ELISA for mono-cultured CLL-cells (CLL Mono), CLL-cells co-cultured with either of the mentioned feeder cells or the feeder cells alone. While NKtert, KUSA and HS5 secreted Ang2 levels comparable to CLL-cells alone, HUVEC supernatant contained about 10fold higher Ang2 levels. Neither of the co-cultures significantly impacted on levels of secreted Ang2. (C) Tie2 surface expression was assessed by flow cytometry after 24h of culture with or without co-culture with feeder cells using a PE-labeled Tie2- antibody. No significant (ns) effect of either co-culture on Tie2 surface levels could be detected. Median fluorescent intensity (MFI) values for Tie2 are summarized for all samples tested (top). One representative histogram for each setting is depicted on the bottom. Significances were calculated using Mann-Whitney U-test |
3.3.3. Hypoxia
- The CLL-cell microenvironment in bone marrow and lymph nodes has been described to feature hypoxic conditions. As hypoxia is a strong stimulus for expression of angiogenic factors in endothelial cells, we assessed the impact of hypoxic conditions on the Ang2- and Tie2-status in CLL-cells (n=5). After 24h supernatant of CLL-cells cultured under hypoxic conditions contained about double as much Ang2 as supernatant of CLL-cells cultured under normoxic conditions with 394 pg/ml +/- 24 pg/ml compared to 197 pg/ml +/- 9 pg/ml, respectively. In HS5-cells hypoxia only lead to a slight, not significant increase in Ang2- secretion (Fig. 5A). Further, the impact of hypoxia on Tie2-expression on CLL-cells was addressed. There was no significant increase in Tie2 surface expression in CLL-cells cultured under hypoxic conditions compared to CLL-cells cultured under normoxic conditions (n=5, Fig. 5B).
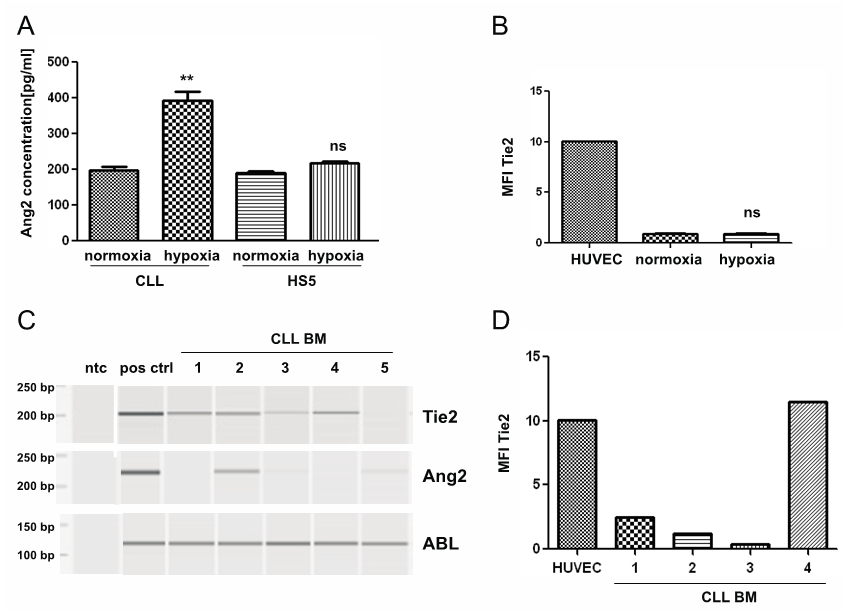 | Figure 5. Ang2 and Tie2 status in hypoxic environment in vitro and in CLL-cells isolated from BM-aspirates of CLL patients: CLL-cells were cultured in normoxia (21% O2) or hypoxia (2% O2) for 24h. (A) Ang2-levels in supernatant of CLL-cells were determined by ELISA. Hypoxia significantly increased Ang2-secretion in CLL-cells (p<0.01), while secretion of Ang2 by HS5 cells was not significantly affected (ns). (B) Tie2 surface expression was assessed by flow cytometry after 24h in normoxia or hypoxia using a PE-labeled Tie2 antibody. No significant difference in Tie2 surface expression could be detected. (C) mRNA was isolated from CLL-cells derived from BM-aspirates of CLL-patients (CLL BM) and converted into cDNA. Tie2 or Ang2 specific primers were used to determine corresponding mRNA-levels by PCR. ABL-specific primers served as housekeeping control. HUVEC functioned as positive control (pos ctrl) for Ang2 and Tie2. A non template control (ntc) served as contamination control. PCR product was analysed by capillary gel electrophoresis. Four out of five CLL-BM samples showed Tie2- expression, while Ang2-expression was heterogeneous. (D) Tie2 surface expression was assessed after erythrocyte lysis of BM-aspirates by flow cytometry using a PE-labeled Tie2- antibody. CLL-cell identification was done by surface staining for CD5 (FITC) and CD19 (PerCPCy5.5). Tie2 surface expression depicted as mean fluorescent intensity (MFI) was comparable to results achieved on the mRNA-level. Significances were calculated using Mann-Whitney U-test |
3.3.4. Primary Bone Marrow (BM)-derived CLL-cells
- While utilized experimental methods to mimic the microenvironment cannot completely resemble the in vivo situation, we assessed the Ang2- and Tie2-status of CLL-cells isolated from BM-aspirates of CLL-patients. The BM features a complex set of stimuli including secreted factors, direct cell-cell contacts as well as hypoxic conditions. Interestingly, four out of five BM-derived samples expressed Tie2 as demonstrated by PCR (Fig. 5C) and flow cytometry (Fig. 5D). Ang2-levels were heterogeneous as assessed by PCR. There was no obvious correlation between Tie2- and Ang2-expression levels in the tested samples (Fig. 5C).
3.3.5. Tie2 Status of Cellular Components Present in the Microenvironment of CLL-cells
- To identify potential targets for CLL-cell derived Ang2, we assessed several cellular components in the microenvironment of CLL-cells for Tie2. We could identify granulocytes as potential target for Ang2 based on their Tie2-positivity (Fig. 6A). Tie2 was slightly upregulated in granulocytes from CLL-patients (MFI 205 +/- 45; n=7) compared to healthy donors (MFI 186 +/- 52; n=11), which was not statistically significant. Figure 6A shows a bar chart summarizing MFIs (left) and representative histograms for granulocytes from CLL-patients and healthy donors (right). To get more insight into the Ang2/Tie2-axis in BM, BM-stroma cells (BMSCs) were generated from BM-aspirates of CLL-patients. PCR analysis revealed Tie2, but no Ang2-expression in primary BMSCs (Fig. 6B). An important cell type in the lymph node, especially in proliferation centers of CLL-patients are nurse like cells (NLCs). NLCs can be generated in vitro by culturing monocytes in the presence of CLL-cells [30]. Neither NLCs, nor CLL-cells cultured on NLCs as feeder layer showed presence of Tie2 as assessed by flow cytometry (Fig. 6C, left and right, respectively).
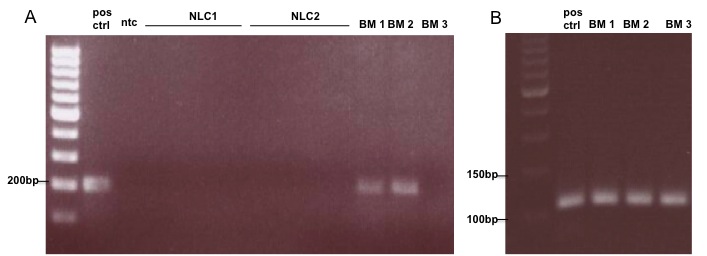 | Figure 6. Tie2 status in CLL-cells of bone marrow aspirates and NLC: CLL-cells and NLC were generated from BM-aspirates from CLL-patients. mRNA was isolated and converted into cDNA. Tie2 specific primers were used to determine corresponding mRNA-levels by PCR. HUVEC functioned as positive control (pos ctrl) for Ang2 and Tie2. A non template control (ntc) served as contamination control. PCR product was analysed by agarose gel. In two of three cases BM-CLL-cells expressed Tie2. Nurse like cells (NLCs) were generated from monocytes in the presence of CLL-cells. They show no Tie2-expression (B) ABL-specific primers served as housekeeping control |
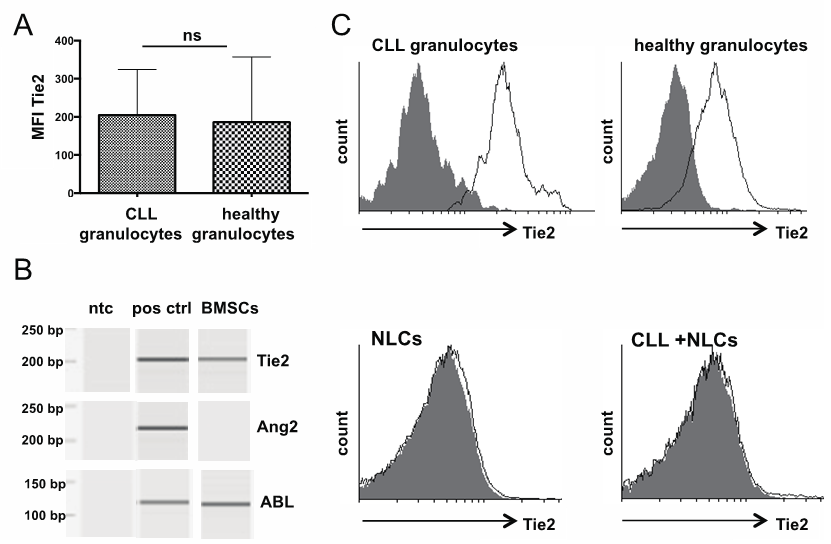 | Figure 7. Tie2 status in cellular components of the CLL-microenvironment: Granulocytes were identified from whole blood of CLL-patients or healthy donors based on positioning in forward and side scatter. Tie2 surface expression was assessed flow cytometrically using a PE-labeled Tie2-antibody. Granulocytes from both, CLL-patients and healthy donors were positive for Tie2 with CLL-cells showing slightly higher mean fluorescent intensity (MFI left). Representative histograms are depicted on the right. (B) BM-stromal cells (BMSCs) were generated from BM-aspirates from CLL-patients. mRNA was isolated and converted into cDNA. Tie2 or Ang2 specific primers were used to determine corresponding mRNA-levels by PCR. ABL-specific primers served as housekeeping control. HUVEC functioned as positive control (pos ctrl) for Ang2 and Tie2. A non template control (ntc) served as contamination control. PCR product was analysed by capillary gel electrophoresis. BMSCs expressed Tie2, but not Ang2 in the one sample analysed. (C) Nurse like cells (NLCs) were generated from monocytes in the presence of CLL-cells. Tie2 surface expression was analysed flow cytometrically using a PE-labeled Tie2-specific antibody. Neither NLCs (left), nor CLL-cells co-cultured with NLCs (right) showed surface Tie2 |
3.3.6. Impact of Ang2 for ME-mediated CLL-cell Survival Support
- To address the question if secreted Ang2 is involved in microenvironment-induced survival support for CLL-cells, we neutralized Ang2 by using the peptibody AMG386 (Trebananib). AMG386 has been described to inhibit interaction between angiopoietins and the Tie2 receptor, thereby blocking downstream effects [10, 31–33]. The dose of 10µg/ml was described to efficiently block angiopoietins [26] and was therefore used in this study. The survival advantage of around 14% CLL-cells gain from co-culture with either HUVEC (n=7) or HS5 (n=10) after 24h remained unchanged when AMG386 was present. AMG386 also did not have any influence on survival of monocultured CLL-cells. Results are presented as summary (left) and as representative dot blots from flow cytometric analaysis of survival based on annexin V-FITC/PI staining.
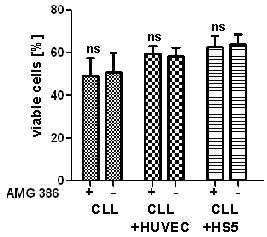 | Figure 8. Impact of Ang2 blockage on CLL-cell survival: CLL-cells were incubated as monoculture or in co-culture with HUVEC or HS5 and treated with the angiopoietin-binding peptibody AMG386 at a concentration of 10 µg/ml for 24h. Cell viability was assessed by flow cytometric measure of Annexin V-FITC/PI. Annexin V/PI-double negative cells were considered viable. Co-culture improved survival by about 10% (compare bars without addition of AMG386). AMG386 did not have an effect on CLL-cell viability, neither in monoculture, nor in co-culture with HUVEC or HS5. A summary is presented on the left and representative dot blots are depicted on the right |
4. Discussion
- The interactions between the leukemic cell and their microenvironments in bone marrow, lymph nodes and peripheral blood are believed to have a significant impact on the pathophysiology of CLL. Increased angiogenesis in the bone marrow has been associated with adverse prognostic markers and aggressive disease [34–36]. The angiogenic factor vascular endothelial growth factor (VEGF) has been described to also possess angiogenesis-independent functions and to be involved in the survival supporting cross-talk between the leukemic cells and their microenvironment [12, 15, 37]. The potent angiogenic factor angiopoietin 2 (Ang2) has also been described to be upregulated in CLL-plasma and correlated with high microvessel density in bone marrow [14, 35]. In AML, the Ang2/Tie2 axis has been suggested to be involved in leukemogenesis and chemosensitivity through a functional role in cell proliferation and survival [22–24]. Up to date the Tie2-status has not been described in CLL and a possible CLL-cell-microenvironment interaction via Ang2/Tie2 has not been studied. Here, we systematically analyzed the Tie2- and Ang2-status in CLL-cells isolated from peripheral blood or bone marrow and the impact of addition of cytokines, co-culture with feeder cell lines as well as hypoxia as common mimics of the microenvironment in an in vitro setting, on the Ang2- and Tie2-status. We also addressed the impact of inhibiting Ang2 by using a blocking peptibody on microenvironment-induced survival support of CLL-cells in vitro.We could confirm upregulation of Ang2 in plasma of CLL-patients compared to plasma from healthy donors. Plasma levels were comparable to published results [21]. Variability of plasma Ang2-levels was associated with the prognostic markers ZAP70 and CD38 (data not shown), thereby published data was confirmed as well [20, 21]. Further, we could demonstrate high Ang2 mRNA levels by PCR in samples which also feature high Ang2 plasma levels. Consequently, also Ang2-mRNA was associated with ZAP70 and CD38 (high levels correlate with ZAP70/CD38 positivity). As tested sample numbers are low, actual correlation analysis was not performed. Comparison of Ang2-levels secreted by CLL-cells into the supernatant and plasma Ang2 (~120 pg/ml vs ~2,500 pg/ml, respectively) suggests either other cell types contribute to plasma Ang2 or the microenvironment stimulates CLL-cells to produce and secrete higher Ang2-levels. To address this we stimulated CLL-cells with IL4 and/or CD40L. Both stimuli improved in vitro survival as expected but did not affect Ang2-secretion. The same was observed for coculture of CLL-cells with bone marrow-derived feeder cell lines: while in vitro survival was improved, Ang2-secretion remained unchanged. Interestingly, Ang2-levels in supernatant of cocultures were around the same concentration of either coculture component alone and not, as expected, in the range of the sum of the two. HS5, KUSA and NKtert Ang2-secretion alone was comparable to Ang2-secretion of CLL-cells alone (all around 200 pg/ml) and also these cocultures showed around the same Ang2-levels in the supernatant. In HUVEC cocultures, Ang2-levels were comparable to HUVEC monoculture alone. This clearly suggests regulation of Ang2-secretion by the microenvironment. Based on high levels of Ang2 in HUVEC/CLL cocultures, which can be suggested to be derived from HUVEC, a possible suppression of Ang2-secretion in CLL-cells by the microenvironment can be suggested. Another microenvironmental factor hypoxia, increased Ang2-secretion in monocultured CLL-cells in our experiments as expected. A more complex mimic of the in vivo situation by combining coculture, which may suppress Ang2-secretion in CLL-cells and hypoxia, which increases Ang2-secretion in monocultured CLL-cells, would be of interest, to get a more detailed insight into this matter.While an involvement of Ang2 has clearly been attributed to CLL-pathophysiology, available data is solely descriptive. Tie2 is the only known receptor for Ang2 and to our knowledge there is no published study addressing the Tie2-status of primary CLL-cells or their microenvironment. We did not detected Tie2 on the cell surface of CLL-cells in peripheral blood. Also stimulation with IL4 and/or CD40L and coculture with feeder cell lines as microenvironment - like stimuli did not induce Tie2 on CLL-cells. In contrast to Ang2, Tie2 wasnot inducible by culture underhypoxic conditions. Hypoxia, as the major trigger for angiogenesis, does not only lead to increased Ang2-production, but also Tie2-upregulation in endothelial cells [38]. As in our experiments, Ang2-production was increased by hypoxia but Tie2-status remained unchanged, it may be concluded that in CLL-cells Ang2/Tie2-signaling does not follow the classical angiogenesis-associated signaling, but may be, at least partially, regulated through other mechanisms. In contrast to CLL-cells from PB, we detected Tie2 on CLL-cells isolated from bone marrow (BM) of CLL-patients. As a consequence of our other results we conclude that microenvironmental factors other than IL4, CD40L, direct physical contact mediated by our coculture systems or hypoxia are responsible for Tie2-expression on BM-located CLL-cells. Based on presence of Tie2 on BM-located CLL-cells, we hypothesize that CLL-cell-derived Ang2 may act in an autocrine fashion in BM, but not in PB. It can further be hypothesized that hypoxic conditions present in the BM may enhance Ang2-production of BM-localized CLL-cells and therefore potentiate autocrine signaling. In hematopoietic stem cells (HSCs) Tie2 was shown to promote adherence and migration in the BM-niche [39, 40], therefore Tie2-positivity in BM-located, but not PB-located CLL-cells seems consequential. Also of interest is the association of Ang2/Tie2 with invasiveness in glioma which was described to involve upregulation of matrix metalloproteinase (MMP) 2 [41]. Also in CLL MMP upregulation has been associated with extravasation of CLL-cells and tissue infiltration, a hallmark of disease progression inCLL [42]. Therefore, is can be hypothesized, that also in CLL Ang2/Tie2plays a role in tissue, BM and/or LN invasion andretaining of tissue localization thereby contributing to promotion of disease progression and aggressiveness.We systematically looked for Tie2-positive cells as potential interaction partnerfor Ang2 present in the CLL-microenvironment. We generated BM-stroma cells from BM-aspirate of a CLL-patient. We detected Tie2-mRNA in BM-derived stromal cells while these cells were negative for Ang2-mRNA. Therefore, BM-stromal cells have the capacity to receive Ang2-signals. Another microenvironment component in CLL are nurse like cells (NLCs), which are mainly present in lymph nodes, but may also be present in BM(43). NLCs were generated in vitro by culturing CLL-PBMCs. Over the time of about three weeks monocytes differentiate in the presence of CLL-cells into a heterogeneous population of large round or epithelial-like adherent NLCs [7]. Neither NLCs themselves, nor cocultered CLL-cells showed Tie2 surface expression. Therefore, NLCs may contribute to Ang2/Tie2-signaling by secretion of Ang2, which was not considered in our experiments. As a possible recipient for Ang2 signals in the microenvironment of the PB, we could identify granulocytes. Granulocytes of healthy donors showed Tie2 surface staining as well, but the signal was slightly increased in granulocytes from CLL-patients. Involvement of granulocytes, which are major players in inflammation, is not surprising considering antigen-stimulation being a critical component in CLL-pathophysiology [44]. Also specialized tissue compartments, so called proliferation centers, found in CLL and known to confer activation of CLL-cells, have also been described in inflamed tissues of patients with autoimmune disorders [45]. Further, a microarray-based gene expression study demonstrated an inflammatory microenvironment to be crucially involved in coculture-mediated survival support [46]. Granulocyte count is usually decreased with increase in disease stage, which contributes to increased susceptibility to infection. An actual involvement of granulocytes in disease progression though is unclear and further studies are warranted in order to make a more clear statement.We also addressed the impact of Ang2 on coculture-induced survival support of cultured CLL-cells by specifically blocking Ang2 using the peptibody AMG386 (Trabananib). Ang2 blockage did not affect survival of CLL-cells in coculture neither with high Tie2-expressing HUVEC, nor moderately Tie2-expressing HS5, suggesting the survival support by these cocultures to be independent on Ang2. To address a possible autocrine effect of CLL-cell-derived Ang2 via an unknown receptor other than Tie2 we blocked Ang2 by addition of AMG386 in a CLL-cell monoculture. As this did not impact CLL-cell survival in this setting, it can be concluded that secreted Ang2 does not feature on autocrine impact on CLL-cell survival.Taken together in the BM, CLL-cell derived Ang2 may act in an autocrine fashion as well as on BM-stromal cells. Also endothelial cells may represent a target for Ang2 in the BM. NLCs do not feature cell-surface Tie2 and may contribute to Ang2/Tie2-signaling in the tissue-microenvironments via Ang2-secretion.Presence of Tie2 on BM-derived, but not PB-derived CLL-cells suggests impact of Ang2/Tie2- signaling on BM-localisation and retention, possibly via involvement of MMPs, and thereby contribute to CLL-progression and aggressiveness. In PB, Tie2 was found on granulocytes
5. Conclusions
- This study contributed to understanding a possible functional role of angiopoietin (Ang) 2, which has been associated with disease progression and aggressiveness in CLL, by delineating potential interaction partners based on Tie2 surface expression in the microenvironments of CLL-cells in bone marrow, lymph node and peripheral blood. This information may assist a more complete understanding of CLL-cell microenvironment interactions, hence allow for development of a therapeutic approach considering the microenvironment.
ACKNOWLEDGEMENTS
- This study was supported by the Wilhelm Sander Foundation, Munich, Germany. The study was further made possible through the biobank of the Center of Integrated Oncology (CIO) Cologne-Bonn funded by the German Cancer Aid, with special reference to Lukas C. Heukamp and Thomas Landwehr.
References
| [1] | Ramsay AD, Rodriguez-Justo M. Chronic lymphocytic leukaemia--the role of the microenvironment pathogenesis and therapy. British journal of haematology 2013 Jul;162(1):15–24. |
| [2] | Buggins AGS, Pepper C, Patten PEM, Hewamana S, Gohil S, Moorhead J, Folarin N, Yallop D, Thomas NSB, Mufti GJ, Fegan C, Devereux S. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer research 2010 Oct;70(19):7523–33. |
| [3] | Hamilton E, Pearce L, Morgan L, Robinson S, Ware V, Brennan P, Thomas NSB, Yallop D, Devereux S, Fegan C, Buggins AGS, Pepper C. Mimicking the tumour microenvironment: three different co-culture systems induce a similar phenotype but distinct proliferative signals in primary chronic lymphocytic leukaemia cells. British journal of haematology 2012 Sep;158(5):589–99. |
| [4] | Audrito V, Vaisitti T, Serra S, Bologna C, Brusa D, Malavasi F, Deaglio S. Targeting the microenvironment in chronic lymphocytic leukemia offers novel therapeutic options. Cancer letters 2013 Jan;328(1):27–35. |
| [5] | Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leukemia & lymphoma 2002 Mar;43(3): 461–6. |
| [6] | Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2008 Sep;26(27):4497–503. |
| [7] | Tsukada N. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood 2002 Feb;99(3):1030–1037. |
| [8] | Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. The Journal of experimental medicine 1992 Nov;176(5):1319–26. |
| [9] | Meinhardt G, Wendtner CM, Hallek M. Molecular pathogenesis of chronic lymphocytic leukemia: factors and signaling pathways regulating cell growth and survival. Journal of molecular medicine (Berlin, Germany) 1999 Feb;77(2):282–93. |
| [10] | Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer research 2013 Mar; 73(6): 1649–57. |
| [11] | Maisonpierre PC. Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts in vivo Angiogenesis Science 1997 Jul; 277(5322):55–60. |
| [12] | Xia Y, Lu R-N, Li J. Angiogenic factors in chronic lymphocytic leukemia. Leukemia research 2012 Oct; 36(10): 1211–7. |
| [13] | Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. 2013;123(8). |
| [14] | Maffei R, Martinelli S, Castelli I, Santachiara R, Zucchini P, Fontana M, Fiorcari S, Bonacorsi G, Ilariucci F, Torelli G, Marasca R. Increased angiogenesis induced by chronic lymphocytic leukemia B cells is mediated by leukemia-derived Ang2 and VEGF. Leukemia research 2010 Mar;34(3):312–21. |
| [15] | Gehrke I, Gandhirajan RK, Poll-Wolbeck SJ, Hallek M, Kreuzer K-A. Bone marrow stromal cell-derived vascular endothelial growth factor (VEGF) rather than chronic lymphocytic leukemia (CLL) cell-derived VEGF is essential for the apoptotic resistance of cultured CLL cells. Molecular medicine (Cambridge, Mass.) 2011 Jan;17(7-8):619–27. |
| [16] | Martinelli S, Maffei R, Castelli I, Santachiara R, Zucchini P, Fontana M, Bonacorsi G, Leonardi G, Marasca R, Torelli G. Increased expression of angiopoietin-2 characterizes early B-cell chronic lymphocytic leukemia with poor prognosis. Leukemia research 2008 Apr;32(4):593–7. |
| [17] | Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harbor perspectives in medicine 2012 Sep;2(9):a006550. |
| [18] | Burger JA. Angiopoietin-2 in CLL. Blood 2010 Jul;116(4): 508–9. |
| [19] | Maffei R, Marasca R, Martinelli S, Castelli I, Santachiara R, Morandi E, Zucchini P, Fontana M, Giacobbi F, Silingardi P, Bonacorsi G, Temperani P, Masini L, Colacci a M, Serra R, Torelli G. Angiopoietin-2 expression in B-cell chronic lymphocytic leukemia: association with clinical outcome and immunoglobulin heavy-chain mutational status. Leukemia 2007 Jun;21(6):1312–5. |
| [20] | Vrbacky F, Smolej L, Vroblova V, Pekova S, Hrudkova M, Cervinka M, Pecka M, Krejsek J, Maly J. Angiopoietin-2 mRNA expression is increased in chronic lymphocytic leukemia patients with poor prognostic features. Hematology (Amsterdam, Netherlands) 2010 Aug;15(4):210–4. |
| [21] | Maffei R, Martinelli S, Santachiara R, Rossi D, Guarnotta C, Sozzi E, Zucchetto A, Rigolin GM, Fiorcari S, Castelli I, Fontana M, Coluccio V, Leonardi G, Zucchini P, Tripodo C, Cuneo A, Gattei V, Del Poeta G, Forconi F, Gaidano G, Torelli G, Marasca R. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood 2010 Jul;116(4):584–92. |
| [22] | Loges S, Heil G, Bruweleit M, Schoder V, Butzal M, Fischer U, Gehling UM, Schuch G, Hossfeld DK, Fiedler W. Analysis of concerted expression of angiogenic growth factors in acute myeloid leukemia: expression of angiopoietin-2 represents an independent prognostic factor for overall survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2005 Feb;23(6):1109–17. |
| [23] | Hatfield KJ, Hovland R, Øyan a M, Kalland KH, Ryningen a, Gjertsen BT, Bruserud Ø. Release of angiopoietin-1 by primary human acute myelogenous leukemia cells is associated with mutations of nucleophosmin, increased by bone marrow stromal cells and possibly antagonized by high systemic angiopoietin-2 levels. Leukemia 2008 Feb;22(2): 287–93. |
| [24] | Hatfield K, Ryningen A, Corbascio M, Bruserud O. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. International journal of cancer. Journal international du cancer 2006 Nov;119(10):2313–21. |
| [25] | Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008 Jun;111(12):5446–56. |
| [26] | Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun J-R, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer cell 2004 Nov;6(5):507–16. |
| [27] | De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell 2005 Sep;8(3):211–26. |
| [28] | Schattner EJ. CD40 ligand in CLL pathogenesis and therapy. Leukemia & lymphoma 2000 May;37(5-6):461–72. |
| [29] | Clark EA, Shu GL, Luscher B, Draves KE, Banchereau J, Ledbetter JA, Valentine MA. Comparison of the Signal Transduced by IL-4 to Four Different Competence. The Journal of Immunology 1989;143(12):3873–3880. |
| [30] | Burger JA, Tsukada N, Burger M, Zvaifler NJ, Aquila MD, Thomas J, Dc W, Burger JA, Tsukada N, Burger M, Zvaifler NJ, Aquila MD, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell – derived factor-1. Blood 2000 Oct;96 (8):2655–2663. |
| [31] | Wu B, Johnson J, Soto M, Ponce M, Calamba D, Sun Y-N. Investigation of the mechanism of clearance of AMG 386, a selective angiopoietin-1/2 neutralizing peptibody, in splenectomized, nephrectomized, and FcRn knockout rodent models. Pharmaceutical research 2012 Apr;29(4):1057–65. |
| [32] | Reikvam H, Hatfield KJ, Lassalle P, Kittang AO, Ersvaer E, Bruserud O. Targeting the angiopoietin (Ang)/Tie-2 pathway in the crosstalk between acute myeloid leukaemia and endothelial cells: studies of Tie-2 blocking antibodies, exogenous Ang-2 and inhibition of constitutive agonistic Ang-1 release. Expert opinion on investigational drugs 2010 Feb;19(2):169–83. |
| [33] | Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, Rasmussen E, Sun Y-N, Zhong D, Hwang YC, Evelhoch JL, Oliner JD, Le N, Rosen LS. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009 Jul;27(21):3557–65. |
| [34] | Letilovic T, Vrhovac R, Verstovsek S, Jaksic B, Ferrajoli A. Role of angiogenesis in chronic lymphocytic leukemia. Cancer 2006 Sep;107(5):925–34. |
| [35] | Kini AR, Kay NE, Peterson LC. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia 2000;14(March):1414–1418. |
| [36] | Molica S. Angiogenesis in B-cell chronic lymphocytic leukemia: methods of study, clinical significance and prognostic implications. Leukemia & lymphoma 2001 Aug;42(4):603–7. |
| [37] | Wang L, Coad JE, Fortney JM, Gibson LF. VEGF-induced survival of chronic lymphocytic leukemia is independent of Bcl-2 phosphorylation. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K 2005 Aug;19(8):1486–7. |
| [38] | Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. The Journal of biological chemistry 2004 Mar;279(13):12171–80. |
| [39] | Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends in cardiovascular medicine 2005 Feb;15(2):75–9. |
| [40] | Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004 Jul;118(2):149–61. |
| [41] | Guo P, Imanishi Y, Cackowski FC, Jarzynka MJ, Tao H-Q, Nishikawa R, Hirose T, Hu B, Cheng S-Y. Up-Regulation of Angiopoietin-2, Matrix Metalloprotease-2, Membrane Type 1 Metalloprotease, and Laminin 5 γ 2 Correlates with the Invasiveness of Human Glioma The American Journal of Pathology 2005 Mar;166(3):877–890. |
| [42] | Kamiguti AS, Lee ES, Till KJ, Harris RJ, Glenn MA, Lin K, Chen HJ, Zuzel M, Cawley JC. The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. British journal of haematology 2004 Apr;125(2):128–40. |
| [43] | Burger J a, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: Insight into disease biology and new targeted therapies. Seminars in cancer biology 2013 Sep. |
| [44] | Brkovic A, Pelletier M, Girard D, Sirois MG. Angiopoietin chemotactic activities on neutrophils are regulated by PI-3K activation. Journal of leukocyte biology 2007 Apr;81(4): 1093–101. |
| [45] | Sachanas S, Levidou G, Angelopoulou MK, Moschogiannis M, Yiakoumis X, Kalpadakis C, Vassilakopoulos TP, Kontopidou F, Tsirkinidis P, Dimitrakopoulou A, Kokoris S, Dimitriadou E, Kyrtsonis M-C, Panayiotidis P, Papadaki H, Patsouris E, Korkolopoulou P, Pangalis G a. Apoptotic and proliferative characteristics of proliferation centers in lymph node sections of patients with chronic lymphocytic leukemia. Leukemia & lymphoma 2013 Jun;(April):1–12. |
| [46] | Schulz A, Toedt G, Zenz T, Stilgenbauer S, Lichter P, Seiffert M. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica 2011 Mar;96(3):408–16. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML