-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2014; 3(1): 1-5
doi:10.5923/j.rct.20140301.01
Anticancer Activity of Copper (II) Complexes with a Pyridoxal-Semicarbazone Ligand
Violeta Jevtovic
Department of Chemistry, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates
Correspondence to: Violeta Jevtovic, Department of Chemistry, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Three newly synthesized copper complexes: [Cu(PLSC)Cl2](1), [Cu(PLSC)(H2O)(SO4)]2.3H2O(2), [Cu2(PLSC)2(NCS)2] (NCS)2 (3) with pyridoxal semicarbazone (PLSC), as ligand, after being subjected to biological tests, showed anticancer activity. As ligand PLSC is biologically active, the results of biological activity are as expected. Specifically, an activity was demonstrated in breast cancer cells (MCF7 and MDA MB 231) and proliferative cells (MCF7). The results suggest that compound 1 exhibit no antiproliferative effect. Compound 2 exhibits cytotoxic effects on both cell lines, but only after 72 h treatment by the highest tested concentrations. Similar cytotoxicity pattern was observed for compound 3.
Keywords: Cu(II) complex, Biological investigation, Anticancer activity
Cite this paper: Violeta Jevtovic, Anticancer Activity of Copper (II) Complexes with a Pyridoxal-Semicarbazone Ligand, Research In Cancer and Tumor, Vol. 3 No. 1, 2014, pp. 1-5. doi: 10.5923/j.rct.20140301.01.
Article Outline
1. Introduction
- Cancer is undoubtedly one of the main health concerns facing our society and one of the primary targets regarding medicinal chemistry. Even though platinum-based complexes had been in primary fo-cus of research on chemotherapy agents[1–3], the interests in this field have shifted to non-platinum-based agents[4–11], in order to find different metal complexes with less side effects and similar, or better, cytotoxicity. Thus, a wide variety of metal complexes based on titanium, gallium, germanium, palladium, gold, cobalt, ruthenium and tin are being intensively studied as platinum replacements[4–11]. Furthermore, copper(II)-based complexes appear to be very promising candidates for anticancer therapy; an idea supported by a considerable number of research articles describing the synthesis and cytotoxic activities of numerous copper(II) complexes[12– 15].The choice of the coordinated ligand(s) seems to be as important as the choice of metal(s) because besides being the integral part of biologically active complexes these organic molecules (ligands) can exert a biological activity of their own[16]. Complexesincorporating3-hydroxy-5-hydroxymethyl-2-methylpyridine-4-carboxaldehyde-3-methylisotios-emicarbazone(pyridoxal-thiosemicarbazone or PLTSC) ligand have not only been in focus of anticancer research in past several years[17–22] but also an inspiration for the synthesis 3-hydroxy-5-hydroxymethyl-2-methyl-4-pyridinecarboxaldehyde semicarbazide or PLSC. Recently, several research articles have been published reporting the synthesis and biological activity of transition metal complexes incorporating PLSC ligand[23–29], including a review article[29] and a PhD thesis[30]. Coordination chemistry of PLSC-based complexes proved to be very interesting as this ligand can exist in neutral, mono- and dianionic forms depending on pH, while its most predominant tridentate coordination mode, achieved through hydrazine nitrogen, phenolic and carbonyl oxygen atoms, allows it to be an excellent chelating ligand[30]. Furthermore, this ligand, based on semicarbazone and pyridoxal moieties (forms found in vitamin B6), has an enormous potential as a biologically active reagent as it has been demonstrated that transition metal complexes incorporating semicarbazones show biological activity. In particular, with regard to biological importance, nickel(II) complexes with semicarbazone ligands show antibacterial activity[31], and copper (II) complexes containing semicarbazones have also displayed biological properties[32–34]. Additionally, several nickel (II) complexes with octadiensemicarbazones exhibit strong inhibitory activity against Staphylococcus aureus and Eschericia Coli[35]. In vitro anticancer studies of several nickel(II) complexes with naphthoquinone semicarbazone and thiosemicarbazone on MCF-7 human breast cancer cells reveal that semicarbazone derivate with nickel(II) complexes is more actively inhibiting cell proliferation than thiosemicarbazone analogues[36]. Thus, we wish to report the anticancer activity of new Copper (II) complexes incorporating a pyridoxal-semicarbazone ligand.
2. Experimental Section
- All commercially obtained reagent-grade chemicals were used without further purification, except for the ligand, which were prepared according to the previously described procedure[37]. The compounds were evaluated for their in vitro cytotoxicity towards two human breast adenocarcinoma cell lines (MCF7 and MDA-MB-231, respectively). Cytotoxic activity was evaluated by colorimetric sulforhodamine B (SRB) assay, after exposure of cells to tested compounds for 24 and 72 hours.The cells were grown in Dulbecco’s Modified Eagle’s Medium with 4.5% of glucose (DMEM, PAA Laboratories) supplemented with 10% foetal calf serum (FCS). Cells were seeded into 96-well microliter plates at the density 5,000 cells/0.1 ml/well, and 24 h after seeding, exposed in triplicate to serial dilutions (100 – 0.4 μM) of samples dissolved in dimethyl sulfoxide (DMSO). Control and blank wells were included in each plate. After 24 and 72 h SRB assay was carried out. The cells were fixed in trichloroacetic acid (TCA) (25 µl of 50% w/v TCA per well) for 1 h at 4°C, washed five times with distilled water, and stained with 50 µl of 0.4% SRB in 1% acetic acid for 30 min. The cells were washed five times with 1% acetic acid and airdried. The stain was solubilized in 10 mM TRIS (pH 10.5), and light absorption was measured using a plate reader (Thermo Labsystems) on 492nm, with reference wavelength 690nm. Cell cytotoxicity was expressed as a percentage of corresponding control value (non-treated cells) obtained in two independent experiments. The original data was analysed by a one-way ANOVA, followed by Duncan’s multiple-range post hoc test. Differences were considered significant at P<0.05. The IC50 values, defined as a dose of compound that inhibits cell growth by 50%, were calculated from concentration- response curves.
3. Results and Discussions
- The reaction betwen 3-hydroxyl-5-hydroxymethyl-2-methyl-4-pyridine-carboxaldehyde semicarbazide (pyridoxal-semicarbazide or PLSC ) and appropriate chloride, sulphate, nitrate or thiocyanate Cu(II) salts in water/alcohol mixtures resulted in the for mation of newcopper (II) complexes: [Cu(PLSC)Cl2](1), [Cu(PLSC) (H2O)(SO4)]2.3H2O(2), [Cu2(PLSC)2(NCS)2](NCS)2 (3). The complexes were characterized by elemental analysis, conductometric measurements and IR spectroscopy, while complexes 1, 2 and 3 were further characterized by single crystal X-ray diffraction (Fig.1)[38].
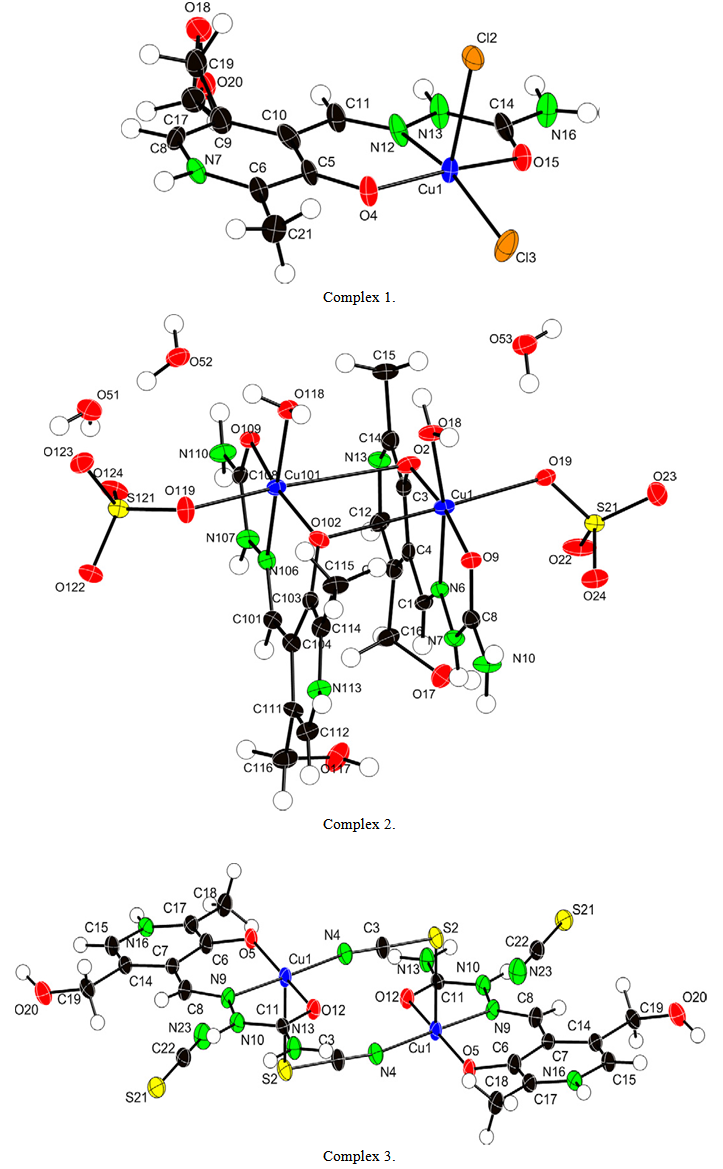 | Figure 1. Molecular structure of the complexe |
3.1. Cytotoxicity Analysis
- Effect of tested compounds on cell proliferation was evaluated using SRB colorimetric assay, based on bonding of SRB compound with total proteins of the living cells[36]. Figure 2 shows effect of different concentrations of tested compounds on two human breast cancer cell lines (MCF7 and MDA MB 231) after 24 and 72 h incubation times.
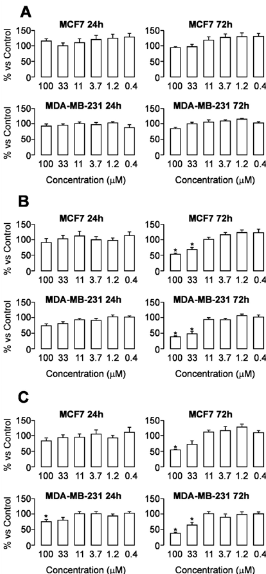 | Figure 2. Effects of compounds 1 (A), 2 (B) and 3 (C) on cell proliferation of MCF7 and MDA-MB-231 human breast cancer cell lines after 24 and 72 h of treatment |
4. Conclusions
- PLSC ligand is biologically active, the results of biological activity are as expected. Cu atom, central atom of complex, is biologically active also. Specifically, an activity was demonstrated in breast cancer cells (MCF7 and MDA MB 231) and proliferative cells (MCF7).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML