-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2013; 2(2): 35-37
doi:10.5923/j.rct.20130202.03
Management of Malignant Fibrous Histiocytoma of Thoracic Wall: A Case Report
Takalkar Unmesh V.1, Asegaonkar Balaji N.1, Asegaonkar Shilpa B.2, Kodlikeri Pushpa R.1, Ujwala Kulkarni1, Suresh H. Advani3
1Kodlikeri memorial’s CIIGMA Hospital Jalna road Aurangabad 431001 Maharashtra India
2Goverment Medical College Aurangabad Maharashtra India
3Asian Institute of Oncology S.L. Raheja Hospital, Raheja Hospital Road, Mahim, Mumbai
Correspondence to: Takalkar Unmesh V., Kodlikeri memorial’s CIIGMA Hospital Jalna road Aurangabad 431001 Maharashtra India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We report management of a case of malignant fibrous histiocytoma (MFH) originated from thoracic wall. A 45 year old man with lump on chest wall was referred to our center. He was diagnosed as MFH histologically. We managed the case with wide optimal surgical resection to negative margin. Then adjuvant chemotherapy and radiotherapy was administered. The patient is disease free since 3 years. MFH commonly arises in soft tissue of extremities and rarely in chest wall. We also reviewed the literature about clinicopathological features, diagnostic modalities and management of MFH. Multidisciplinary approach of surgical resection followed by chemo radiotherapy result in better outcome.
Keywords: Malignant Fibrous Histiocytoma, Thoracic Wall, Wide Excision, Chemotherapy, Radiotherapy
Cite this paper: Takalkar Unmesh V., Asegaonkar Balaji N., Asegaonkar Shilpa B., Kodlikeri Pushpa R., Ujwala Kulkarni, Suresh H. Advani, Management of Malignant Fibrous Histiocytoma of Thoracic Wall: A Case Report, Research In Cancer and Tumor, Vol. 2 No. 2, 2013, pp. 35-37. doi: 10.5923/j.rct.20130202.03.
1. Background
- Malignant fibrous histiocytoma (MFH) is a common, aggressive soft tissue sarcoma of mesenchymal origin. It occurs commonly in late adult life and rarely does it involve the chest wall. Generally it is located as mass of extremities (70%), abdominal cavity or retroperitoneum (16%) involving deep fascia, skeletal muscle or superficial subcutis[1]. Herein we described a rare case of primary MFH originating from chest wall with purpose of examining clinicopathological features. Also present case report emphasizes successful management of. MFH with multidisciplinary approach for better outcome and frequent monitoring. Case presentation: A 45 year old policeman was referred to our center with chief complaint of lump on right chest wall. He presented with 4 month history of progressively enlarging painless mass. On detailed acquisition of history he was nonsmoker, nonalcoholic. He was tobacco chewer since last 10 years. He had no significant past medical and surgical history suggestive of any major disorder. He was never exposed to radiotherapy or any injury, scar over chest wall. Physical examination revealed huge oval shaped mass of size 10 x 8 cm on right side of chest wall as shown in figure 1. The mass was firm on palpation and not fixed to chest wall. Skin over the lump was tethered in the centre. Margins of the mass were diffuse and mobility was restricted. We thought of differential diagnosis of metastasis from other primary lesion or multiple myeloma for this condition. We ruled out these possibilities with aid of various diagnostic modalities. Ultrasound examination suggested presence of solid lobulated mass of soft tissue lipoma or liposarcoma. Fine needle aspiration cytology revealed presence of malignant cells with provisional diagnosis of liposarcoma. Routine laboratory work up was within stipulated range. As tumor markers are not specific for chest wall tumors we did not advised them for analysis. Patient underwent diagnostic tests to detect presence of local and distant metastasis.
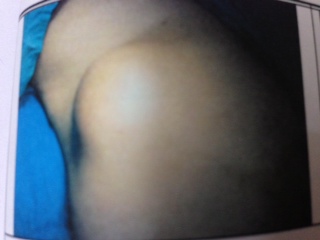 | Figure 1. Chest wall lump on right side |
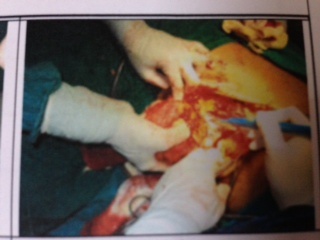 | Figure 2. Wide surgical resection of mass on right chest wall |
 | Figure 3. Gross specimen of resected mass from chest wall |
2. Discussion
- MFH, the most common aggressive tumor of soft tissue rarely originates in chest wall. Common sites affected by MFH are extremities, abdominal cavity and retroperitoneum. Diagnosis of MFH was introduced first in 1964 by O’Brien and Stout as soft tissue sarcoma composed of fibroblast-like and histiocytic-like cells, mixed with pleomorphic giant and inflammatory cells.[2] Its subtypes described are storiform- pleomorphic, myxoid, giant cell rich, angiomatoid and inflammatory MFH. Predisposing factors for causation of MFH are prior exposure to radiation, asbestos, and scar of trauma, burn and surgical incision[3]. But in our case no such risk factor identified.Maze Y and colleagues reported a case of MFH of chest wall managed by wide resection of tumor and partial lung[4]. Sawai H et al reported 31% local recurrence, 9% distant metastasis and 36.1% deaths within first year of diagnosis in patients of MFH managed with surgical resection[5]. Early diagnosis of MFH is crucial as it tends to enlarge to huge size within short period of time if neglected. Christophoros described giant myxoid MFH of chest wall of size 34 cm weighing 6.9 Kg in a 79 year old woman[6]. There is scarcity of trials regarding addition of chemotherapy and radiotherapy in MFH cases. Bielack and associates analyzed 125 cases of MFH of bone retrospectively in their EMSOS study. They reported more favourable outcome in patients with younger age and use of chemotherapy[7]. Kitazono described long term survival of a 70 year old man with MFH of chest wall involving ribs followed by local recurrence. He received multidisciplinary treatment of resection for each metastasis with addition of radiotherapy (66 Gy) and chemotherapy[8]. Yasunori and colleagues described radical en-block resection for MFH of chest wall[9]. Sharma P et al reported a case report regarding MFH of chest wall masquerading as medullary breast carcinoma. Initially this patient was diagnosed as medullary carcinoma of breast. She received neo-adjuvant chemoradiotherapy before resection of tumor. But with immunocytochemical stain diagnosis of MFH was established[10]. More than 50% of chest wall tumors are either primary malignant lesions or secondary lesions[11]. With the advances in diagnostic modalities and surgical techniques, wide excision has resulted in long term survival with reduced recurrence rate. Chest radiography, computed tomography, magnetic resonance imaging and positron emission tomography give an account about exact location, size, extent, composition and vascularity of tumor in chest wall. Fine needle aspiration cytology and biopsy help in tissue diagnosis. This preoperative investigation work up helps to decide management strategy. Complete medical evaluation especially with careful assessment of cardiac and pulmonary function is mandatory before wide excision of tumor from thoracic wall. Local recurrence rate of MFH is more than 30%, metastatic lesions in 30-50% cases with only 38% 5 year survival rate. To avoid local recurrence wide excision to negative margins is the mainstay in management of MFH[11]. Most of the reports suggest poor prognosis of primary MFH because of its biologically aggressive nature. Tumor grade, size, depth, vascular invasion and presence of metastasis remain the most important prognostic factors for MFH. Resection with negative microscopic margins and adjuvant chemotherapy decreases local recurrence of tumor [12, 13]. Lifelong surveillance with physical examination and imaging investigation is key element in the management of MFH. We offered adjuvant chemotherapy and radiotherapy to our patient which contributed for disease free state since 3 years.After careful review of previous literature and present case report of MFH of chest wall, we consider adequate surgical resection of tumor followed by adjuvant chemotherapy and radiotherapy remains treatment of choice to obtain favorable outcome.Conclusion: The present case is unique because of location of tumor in thoracic wall. Radiographic imaging techniques, histopathological examination along with immunohistochemistry help in establishing diagnosis of MFH. Wide surgical resection of tumor with additional chemotherapy and radiotherapy contributes to long disease free survival state. High recurrence rate emphasizes importance of routine follow up in the management of MFH.Conflicts of interests: There is no financial or any other conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML