-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research In Cancer and Tumor
2013; 2(1): 1-9
doi:10.5923/j.rct.20130201.01
The Nexus between Interdisciplinary Approach and Extended Survival in CNS Tumors, Neuro-Oncology Scientific Club (NOSC)Meeting Report, 27 December 2012, Isfahan, Iran
Alireza Amouheidari1, Simin Hemati2, Masih Sabouri3, Jafar Emami4, Valiollah Mehrzad5, Ali Hekmatnia6, Behzad Alian2, Hoda Rouhani Najafabadi7, Mohammad Torabi Nami7
1Department of Radiation-Oncology, Isfahan Milad Hospital, Isfahan, Iran
2Department of Radiation-Oncology,School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
3Department of Neurosurgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
4Emeritus Faculty, Department of Radiation-Oncology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
5Division of Hematology-Oncology, Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
6Department of Radiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
7Behphar Scientific Committee, Behphar Group, Tehran, Iran
Correspondence to: Mohammad Torabi Nami, Behphar Scientific Committee, Behphar Group, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The Neuro-Oncology Scientific Club (in short, NOSC) is a local initiative by Iranian neuro-oncology experts who have particularly been interested in optimal management of brain tumor patients. Since October 2011, NOSC’s serial meetings in Iran major provinces have focused on executive strategies to improve brain tumor care through an interdisciplinary approach. The current report outlines the communicated insights and agreed-upon decisions during the first Isfahan NOSC meeting held on 27th December 2012 in Isfahan, Iran. Evidence and local trends on maximal safe resection to improve outcome in gliomas with its pitfalls and current solutions, the practical medical considerations in glioma management and the issue of recognizing glioblastoma multiforme pseudoprogression to avoid imposing a wrong premature stop in chemotherapy were discussed during this event. The well-structured brain tumor collaborative registry (BTCR) software which had already been developed by the parallel working-groups within NOSC, was introduced during the session. NOSC members, collaborators and consultants shared ideas to arrive at a common place for the vision, mission and forthcoming plans of this newly established scientific club in Isfahan. They agreed to use this platform to explore opportunities for shared research as well as integrated diagnostic and therapeutic approaches in order to further provide Isfahan brain tumor patients with the best possible care. The provincial brain tumor epidemiological updates, progress report in radiodiagnostic measures and dataset review from the first phase data gathering through BTCR, will be the main constituents of the next Isfahan NOSC meeting to be held early June2013.
Keywords: Neuro-Oncology, Interdisciplinary, Guideline Definition, Brain Tumour, NOSC, Isfahan
Cite this paper: Alireza Amouheidari, Simin Hemati, Masih Sabouri, Jafar Emami, Valiollah Mehrzad, Ali Hekmatnia, Behzad Alian, Hoda Rouhani Najafabadi, Mohammad Torabi Nami, The Nexus between Interdisciplinary Approach and Extended Survival in CNS Tumors, Neuro-Oncology Scientific Club (NOSC)Meeting Report, 27 December 2012, Isfahan, Iran, Research In Cancer and Tumor, Vol. 2 No. 1, 2013, pp. 1-9. doi: 10.5923/j.rct.20130201.01.
Article Outline
1. Introduction
1.1. Neuro-Oncology and its Interdisciplinary Nature
- While treatment of high grade glioma (HGG) and the expected outcome has remained a big medical and surgical challenge since many years[1], the outstanding advances in molecular biology, diagnostic measures, surgical techniques, radiotherapy protocols and drug development have allowed a relatively extended survival and better quality of life in brain tumor patients[2]. The so far studies with their most clinically relevant achievements are turning neuro-oncology to a viable and fast-growing area of basic and clinical research. Implementation of coordinated and well-driven care to brain tumor patients is expected to result in more favorable outcomes[3]. This cannot happen without an interdisciplinary approach which cements the links between specialties involved in management of brain tumors.
1.2. The Neuro-Oncology Scientific Club
- The Neuro-Oncology Scientific Club (NOSC) started its journey about a year ago. Iranian experts from brain tumor allied disciplines who have the shared aim of “serving brain tumor patients better”, decided to unite within this interdisciplinary scientific club since October 2011.The vision and mission of NOSC has provided a common place to bridge neuro-oncology related specialties and strengthen partnership to provide brain tumor patients with a better care.Furthermore, NOSC tries to cater the needs of scholars and specialists with brain tumor interest such as radiation oncologists, hem-oncologist, radiologists, neurologists, pathologists, etc. through high quality educational case studies, reports and presentations during the interval (seasonal) sessions in different provinces across Iran. Adding to the above, NOSC will pursue its aim through: 1- acting as a provincial and later on, national guideline definition group for brain tumor diagnosis, management and follow up; 2- reporting interesting brain tumor cases of educational value (NOSC case study periodical); and 3-organizing patients’ awareness campaigns and support programs. The so far documented and published reports from NOSC activities may be considered as a common pool for all clinicians and researchers in the field to review local experiences as well as recommended solutions for optimal management of brain tumor patients [4-6].
1.3. Isfahan NOSC
- The present report outlines the outcome of the first Isfahan NOSC meeting held on 27th December 2012 in Isfahan, Iran. Bringing some central issues into question, the panel and contributors/consultants of this meeting debated achievements vs. drawbacks in neuro-oncology practice in this province. They defined where NOSC is going to reach? Moreover, maximal safe resection to improve outcome in gliomas with its pitfalls and current solutions, the practical medical considerations in glioma management and the issue of recognizing glioblastoma multiforme pseudoprogression to avoid imposing a wrong premature stop in chemotherapy were discussed during this interactive session. NOSC possesses a well-structured collaborative brain tumor registry[7]. The content of this registry has been developed by parallel working-groups from its within. This software got introduced to Isfahan NOSC members during the meeting.
2. Diagnostic Modalities, Treatment Strategies and Follow up in Brain Tumours
2.1. Maximal Safe Resection to Improve Outcome in Gliomas; Pitfalls and Current Solutions
2.1.1. Discussion
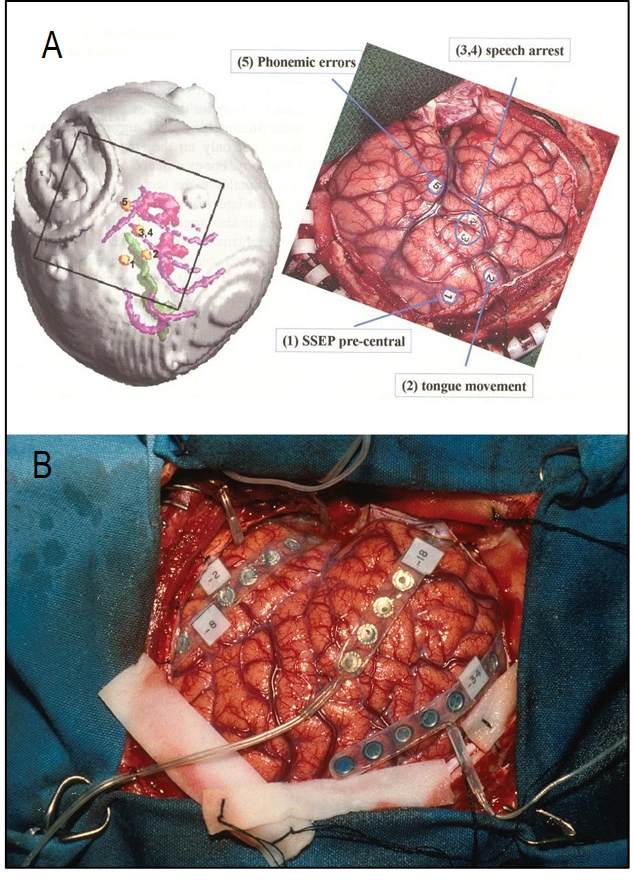
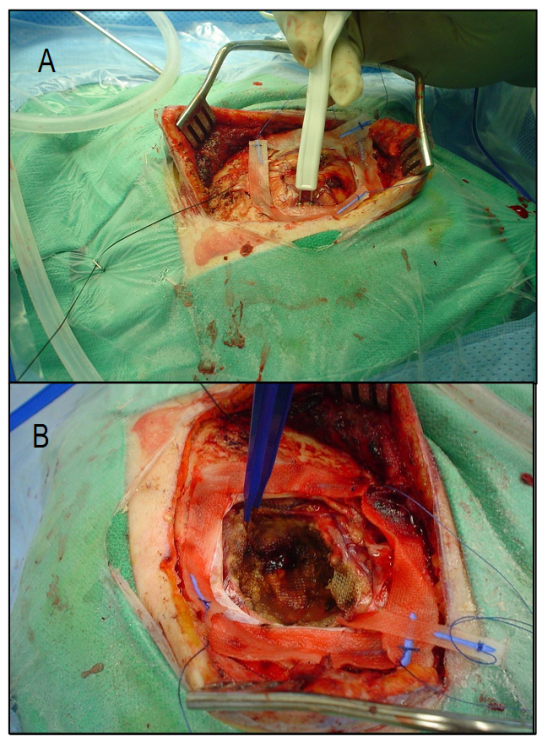
- Malignant gliomas are the most frequent primary brain tumors. Despite medical and surgical advances in HGG management, survival rate at 2 years remains trivial[10]. The benefits of maximal safe resection and its effectiveness on survival is no longer debated[10, 11]. However, HGG is a highly infiltrative tumor thus cannot be totally resected in most instances[11]. Given this, even resected tumors may have microscopic infiltrates potentially leading to recurrence. Some favorable prognostic factors such as younger age (20-40 years), Karnofsky performance status (KPS) rating score>70, and the extent of resection define the better outcome in GBM[12,13]. Surgical approaches include maximal safe resection (total or subtotal tumor removal), biopsy or debulking ± BCNU wafers. These implants (with sustained delivery of the chemotherapy agents to tumors) were first tried in recurrent GBM cases and showed to increase the overall survival(OS). Using BCNU wafers in initially diagnosed GBM patients has shown survival benefits and is currently approved by FDA[14, 15].Following the advent of stereotactic method, less invasive biopsy taking became feasible. This method is now being widely employed in our setting. Knowing the histopathological characteristics of the tumor would guide the whole treatment team towards more precise executive decisions for each individual case. Although survival is shown to be improved following radical resection, potential sequelae following surgery should be seriously taken into account[16]. The surgical trajectory and the technique used would help minimizing the risk of harming eloquent brain areas.The most popular methods to improve radical resection outcome include functional MRI, intraoperative electrophysiological monitoring, ultrasound imaging, image-guided surgery, metabolic imaging and awake craniotomy[16,17].Pre-surgical functional map of the brain using fMRI or PET will allow the surgeon to have a clear planning. Functional MRIdata of the eloquent brain can be integrated for intraoperative navigation to minimize the damage to functionally critical areas of both cortical and subcortical pathways. Figure.1 demonstrates speech fMRI data integrated for navigation. Intraoperativeelectrocorticography (ECoG) and somatosensory evoked potentials (SSEPs) will assist obtaining simultaneous functional data while operating in the awake craniotomy setting. Electrographic phase reversal in the electrophysiology tracing delineates the boundary between motor and sensory cortices and the areas functionally involved when the patients carry out the task during awake craniotomy. SSPE is induced by bipolar stimulation probe (Ojemann) at 1ms, 60 Hz and 2-18 mA. By this, we make sure if the motor cortex for instance, remains functional following the resection (Figure2). Mapping of cognitive functions would be done by electric stimulation-induced transient disturbances in language, calculation, comprehension, memory, writing, etc. Possible impairments are simultaneously communicated with the surgeon when he touches the corresponding critical areas. Based on the clinical neuroscientist’s inputs during the operation session, sterile numbered tags will be applied on relevant cortical zones, assisting the surgeon to avoid damaging the marked eloquent regions (Figure 1).
2.1.2. Conclusion and Recommendations
- Safe maximal resection of brain tumors is a critical issue to be addressed in neurosurgery practice. There are novel evolving techniques assisting the surgeons to save eloquent cortices when operating on the tumor. These comprise electrocortical stimulations (ECS) and ECoG recordings. Intraoperative navigations by frameless stereotaxy would help even more precise approach when excising tumors[17]. Noting the impact of safe maximal resection of the brain tumor on patients’ survival, the use of awake craniotomy and functional surgery set ups is strongly recommended by experts Isfahan is now one of the leading cities to have awake craniotomy setup. There are continuing efforts to soon install the intraoperative image-guided surgery, ECoG and awake craniotomy facilities in Iran.
2.2. Neuro-oncology Today; the Practical Considerations in Glioma Management
2.2.1. Discussion
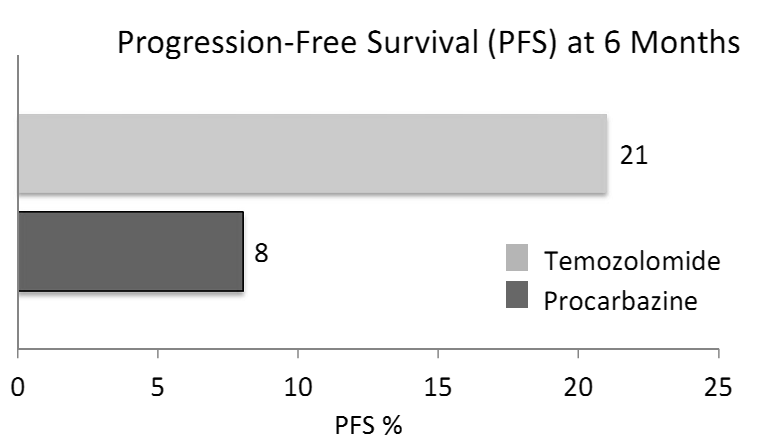
- Radiotherapy has been a therapeutic measure for GBM since 1970s[18]. However,about a decade ago Stupp et al. showed that addition of concomitant and adjuvant temozolomide (TMZ) can increase the GBM overall survival (OS) up to 15 months[19] (Figure 3). In 2005, the EORTC/NCIC (European Organization for research and Treatment of Cancer/National Cancer Institute of Canada) trial data indicated that the median OS in newly diagnosed GBM (WHO PS 0-1) undergoing RT/TMZ vs. RT alone is 14.6 and 12.1 month, respectively. Further follow up data in 2009 revealed that over a median follow up of 61 months, 9.8% vs. 1.9 % ofRT/TMZ and RT alone arm patients attain a 5 year survival, respectively[19, 20]. Molecular studies have shown that epigenetic silencing of DNA repair enzyme MGMT (O6-Methylguanine-DNA-Methyl-Transferase) plays a predictive role in GBM survival. The median OS for methylated vs. unmethylated MGMT has been reported 21.7 and 15.3 months, respectively[20-22].Evidence has shown that radiosurgery[23] and brachytherapy boosts[24]provide no added survival benefits. Moreover, outside the EORTC/NCIC protocol for GBM chemoradiotherapy, efforts to optimize TMZ dosing (e.g. dose dense regimen) have led to no significant difference in OS as compared to TMZ standard dosing protocol. These strategies on the other hand, have increased the rate of chemotherapy-related adverse events such as lymphopenia and fatigue[25].EORTC/NCIC study protocol is now being used as the most widely accepted paradigm in GBM management. In this protocol, radiotherapy consists of fractionated focal irradiation at 2 Gy/fraction once daily, 5 days/weekfor a total of 60 Gy over 6 weeks. Radiotherapy is delivered to the gross tumor volume with a 2- to 3-cm margin to account for the clinical tumor volume. After a 4-week break, patients should receive 6 cycles of maintenance TMZ on the standard 5-day schedule every 28 days. The adjuvant TMZ dose of 150 mg/m2 for the first cycle is to be escalated to 200 mg/m2 from cycle 2 onward in the absence of severe (grade 3 or 4) hematological toxicity[19] (Figure 3).With regard to MGMT, as best as we can determine today, the analysis of this enzyme’s status is prognostic but not predictive meaning that while MGMT does not predict the treatment outcome, it is of high prognostic quality. At present, guidelines do not suggest a different treatment strategy for methylated MGMT GBMs, therefore its routine assessment is not recommended. However there is often a drive to investigate MGMT methylation status as it is shown to partly define treatment strategies in some patients[22, 26].Despite the success of concurrent and adjuvant TMZ, tumor progression after therapy remains the rule and cytotoxic chemotherapy may have an important role in the treatment of recurrent GBM.Phase II trials of the combinations of procarbazine(PCB) and fotemustine as well as BCNU and irinotecan (CPT-11) in the treatment of patients with recurrent GBM following TMZ therapy have suggested anti-tumor activity[27].Irinotecan is also often used in combination with bevacizumab (BCZ), an anti-VEGF(vascular endothelial growth factor) therapy [28, 29].In a randomized active-controlled phase II clinical trial, it was shown that in patients with recurrent gliomas, 21% of those treated with TMZ demonstrate a progression-free survival versus only 8% for those treated with the active control, procarbazine. Median progression-free survival was 2.89 months in the TMZ patients vs. 1.88 months in the procarbazine group, and the proportion of patients surviving at 6 months was 60% in the TMZ group vs. 44% in the procarbazine group[30] (Figure4).
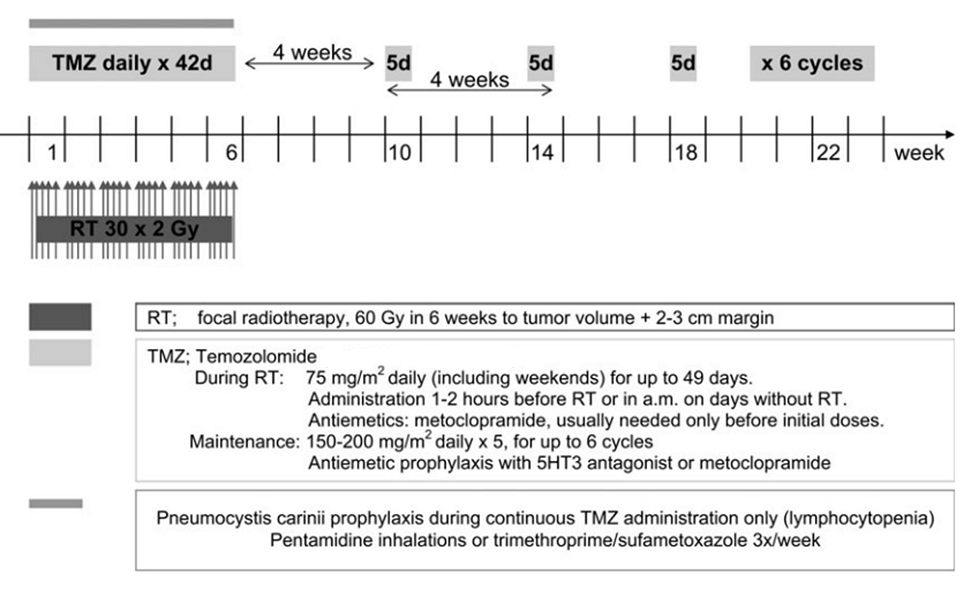 | Figure 3. Pivotal trial of temozolomide: the treatment regimen protocol[19] |
2.2.2. Conclusions and Recommendations
- Based on the discussed evidence, the current paradigm of non-surgical therapy in high-grade glioma and GBM in particular, remains the standard concurrentradio-chemotherapy followed by adjuvant chemotherapy using TMZ according to Stupp et al. protocol [19].Among these lines, fractionated stereotactic radiosurgery (SRS) has shown to render survival benefits in recurrent GBM (median OS of 7-14 months after recurrence)[31].At cellular level and in light of new drug developments, targeted therapies which aim at redundant pathways such as tyrosine kinase inhibitors, MAPK ( membrane associated protein kinase) pathway inhibitors and those targeting PI3K,Akt and mTOR pathway (an intracellular signaling pathway important in apoptosis and hence cancer) are expected to offer further hopes in GBM management[2].
2.3. Recognizing GBM Pseudoprogression or Imposing a Wrong Premature Stop in Chemotherapy?
2.3.1. Discussion
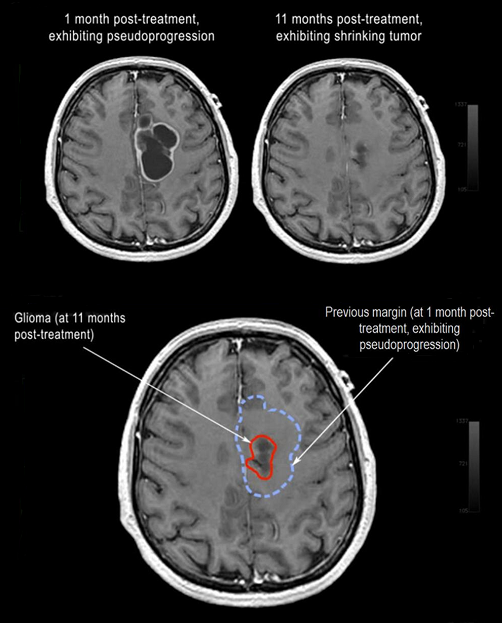
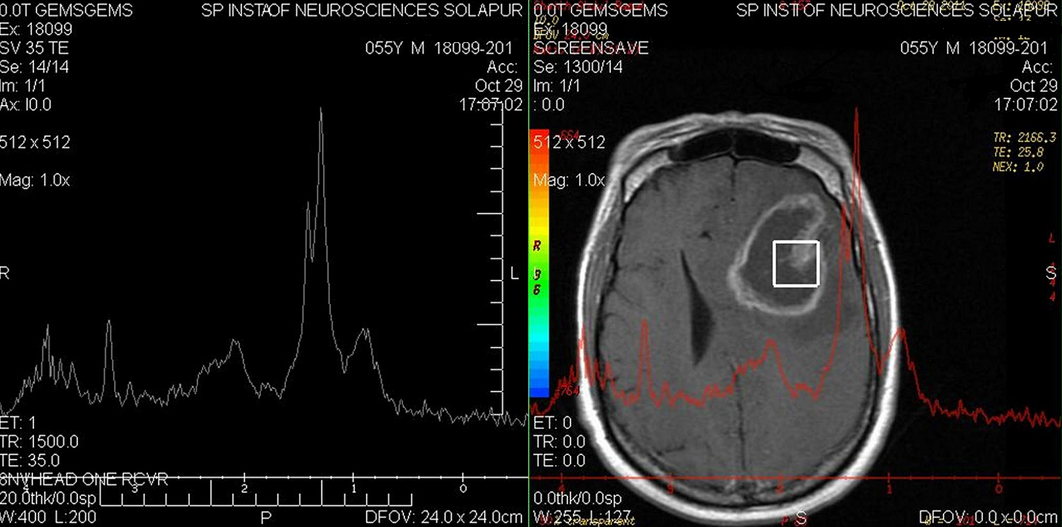
- One of themain signs of anatomical changes in tumor progression includes limitation in delineating the full extent of tumor. Radiographic changes in MRI are weeks or months behind the molecular changes[32]. It should be noted that early post-RTchanges (peri-tumoral edema, inflammation and microvascular changes or necrosis) may mimic progression[32]. This condition which is known as pseudoprogression is defined as a transient increased contrast enhancement found on an MRI scan that can mimic tumor progression but eventually subsides without any change in therapy. It is estimated that pseudoprogression is identified in approximately 20% to 30% of patients suspected of demonstrating early progression (Figure 5).Currently, there are no available radiologic methods that can definitively differentiate between pseudo- and true progression[32, 33].Pseudoprogression cannot be distinguished by CT or MRI alone, however, diffusion-weighted MRI, MRS ( Magnetic Resonance Spectroscopy) and PET ( Positron Emission Tomography) imaging may be helpful. Figure 6 shows a MRS graph corresponding to T2 MR image. T2 shows an iso-intense thick enhancing wall with a hyper-intense central non-enhancing necrosis. The radiologist has been eager to know if the nodular portion of the lesion retains the chemical characteristics of true- vs. pseudoprogression. He thus chose a 2x2cm voxel over the nodular portion of lesion and obtained a spectral waveform in 35 ms, showing sharp doublet of lactate ( a surrogate for necrosis) at 0.9 to 1.3ppm, short peaks for markedly reduced NAA(N-Acetyl Aspartate) at 2.02ppm and creatinine at 3.02ppm. The above findings are in favour of pseudo-progression of the tumor (Figure 6).Based on the most recent Canadian guideline in GBM management, when imaging fails to provide conclusive data, continuation of adjuvant chemotherapy and re-imaging after three cycles is recommended. PET scan is also considered as an appropriate imaging modality forthe above purpose [32-34].As outlined by the Response Assessment in Neuro-Oncology Working Group (RANO)[35], the criteria for determining progression are based on the time from initial radio-chemotherapy. These criteria for determining progression are a working group’s international effort to recommend updated standardized response criteria in clinical trials for high grade gliomas. The new consensus criteria need to be developed and validated in future clinical trials. The following criteria provide guidance on the types of radiographic findings that are suggestive of progressive disease [35]:a. Less than 12 weeks from completion of radiochemotherapy;Progression is defined as a new enhancing lesion outside of the radiation field (beyond the 45-Gy dose line) or as unequivocal evidence of viable tumor on the histopathology sampling.b. 12 weeks or more from completion of radiochemotherapy;A new contrast-enhancing lesion present on the outside of the radiation field while the patient is on decreasing, stable, or increasing doses of a corticosteroid constitutes progression. Another criterion for progression is a ≥25% increase in the sum of the products of perpendicular diameters between the first post-radiotherapy scan, or a subsequent scan with smaller tumor size, and the scan at 12 weeks or later on stable or increasing doses of corticosteroids.Clinical deterioration not attributed to concurrent medications or to comorbid conditions is also a criterion for progression[35].To differentiate pseudoprogression from true progression, clinical deterioration of the patient and imaging may partly help. Other diagnostic tools which may help include sequential MRI assessments; MRS, diffusion weighted MRI (DW-MRI), dynamic perfusion permeability MRI, PET scan (preferably using methionine rather than glucose as the radiotracer) and SPECT[35].
2.3.2. Conclusions and Recommendations
- Following discussions during Isfahan NOSC, we decided to designate at least one academic radiodiagnostic facility in the province to provide special imaging modalities (including MRS) to help delineate pseudo- from true progression in brain tumors. Watchful waiting plus corticosteroids are now considered a proper approach when we face the question of pseudoprogression in HGGs. Adjuvant chemotherapy would not be stopped for at least three consecutive cycles unless clinical deterioration occurs and evidence strongly suggests the recurrence.
3. Achievements vs. Drawbacks ofNeuro-oncology Practice in Isfahan, Where does NOSC Plan to Reach?
3.1. Discussion
- Patients with brain tumor presenting-symptoms are appropriately referred for standard imaging. Radiological impression would put forward the idea of tumour biopsy and surgery when indicated. In some instances however, where debulking or resection is neither feasible noran option, patients only undergo the stereotactic biopsy.Isfahan is known to have one of the most equipped and organized neurosurgery setups in Iran. Over 400 brain tumor cases have undergone stereotactic tumor biopsy over the past years. Likewise, many patients have had the chance of maximal safe resection under traditional techniques or awake craniotomy/functional surgery. Where pathology investigation reveals the diagnosis; neurology, psychiatry, nursing, etc. render the corresponding care andradiation oncology plays its central part offering the medical standard of care. For high grade gliomas (HGG) and glioblastoma in particular, the standard of care remains radiotherapy(RT) plus concomitant and adjuvant temozolomide (TMZ) [8, 9]. Hematology-oncology also serves HGG patients either in primary or recurrent settings.Neuro-oncology practice in Isfahan has enjoyed some achievements over the past years. These include: 1-the relative working team spirit has allowed at least a handful number of HGG patients become long-term survivors (overall survival >4 years); 2- the current approach in maximal safe resection followed by the standard of care is generally helping many of our HGG patients to live longer and better quality lives as compared to past and 3- over recent years, the multileaf collimator (MLC)-equipped linear accelerators have been installed in Isfahan. Given the current availability of CT-based treatment planning and the automatic radiation delivery systems, our patient have access to modern radiotherapy techniques. Despite the above, there are some drawbacks hindering further achievements. They are the followings: 1- as compared to other cancers, CNS malignancies have less been of our focus in academic round tables, seminars and congresses, hence brain tumor translational research has not received the deserved attention; 2- we still lack advanced radiological technologies such as magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI) and positron emission tomography (PET), moreover, diffusion- and perfusion –weighted MRIs are not routinely performed in Isfahan facilities; 3- we still need to improve the crosstalk between neurosurgery and radiation oncology to let patients be timely referred to receive the standard of care if their clinical status allows; 4- written communication among the treating physicians should include as much clinical data and descriptions as possible, this would optimize the medical records; and 5-a comprehensive local consensus algorithm ( guideline driven)to unify our practice is lacking.
3.2. Conclusions and Recommendations
- 1-within Isfahan NOSC, the communicated updates and evidence-based inputs will be incorporated to a local consensus algorithm, primarily for the management of HGG; 2-Isfahan NOSC will have tissue bank of obtained brain tumour biopsies over which histopathological, cellular and molecular investigations will be possibly designed and implemented; 3- the NOSC periodical meetings and share of experience are expected to help providing optimal care to the challenging brain tumor cases thus would be a major step to overcome some of the current pitfalls in patients’ management.
4. Prospective Improvements for the Neuro-Oncology Scientific Club
- NOSC members, collaborators and consultants shared ideas to arrive at a commonplace for the vision, mission and forthcoming plans of this newly established scientific club in Isfahan. Isfahan NOSC would try to foster a team-work in diagnosis, treatment and follow up of brain tumor patients. Below are the shared decisions made during the meeting.1) Everyone agreed with NOSC’s rationale, vision and mission and the idea of having periodical meetings for the exchange of experience to achieve its defined goals.2) NOSC’s ultimate aim will be improving our brain tumor patients’ health and quality of life through an interdisciplinary team-work. 3) Participation of expert physicians/scientists from all allied disciplines should be further encouraged. 4) The final version of the collaborative brain tumor registry (CBTR) which is already developed by NOSC’s parallel working-groups,will be installed in designated cancer care centers in Isfahan. This would help a more organized brain tumor data gathering within the province. 5) Within NOSC, the communicated updates and evidence-based inputs will be incorporated to a local consensus algorithm, primarily for the management of HGG.6) NOSC will contribute to organize and conduct “CNS tumor boards” in Isfahan. Interesting cases will be reported in NOSC case study periodical and subsequently published in its dedicated website.7) The forthcoming Isfahan NOSC meeting will be in early June 2013. Provincial brain tumor epidemiological updates, progress report in radiodiagnostic measures, dataset review from the first phase data gathering through BTCR, will constitute the main part of the next meeting’s agenda.
ACKNOWLEDGMENTS
- Authors would like to thank DrsP. Dindoust, S.A. Hejazi Farahmand, L. Nafarieh, F. Mohammadzadeh (Behphar Scientific Committee, Behphar Group, Tehran) and L.Mukhomorova(MSD), for their organizational/scientific support to this meeting.
Disclosure
- The present report outlines the communicated insights and agreed-upon decisions during the first Isfahan NOSC meeting held on 27th December 2012 in Isfahan, Iran. This meeting received organizational and scientific support from Behestan Darou PJS, Tehran, Iran. Authors declare no conflict of interest upon data review, talk delivery during the meeting and the preparation of this report. The co-authors HRN and MTN are affiliated to Behphar Scientific Committee, Behphar Group, Tehran, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML