-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2023; 13(1): 43-51
doi:10.5923/j.plant.20231301.03
Received: Nov. 20, 2023; Accepted: Nov. 30, 2023; Published: Dec. 13, 2023

Enrichment of Extract Composition of Merzifon Karası Grape Cultivar with MeJA Elicitor
Emine Sema Çetin, Ali Tufan Erşenel
Yozgat Bozok University, Faculty of Agriculture, Department of Horticulture, Yozgat, Türkiye
Correspondence to: Emine Sema Çetin, Yozgat Bozok University, Faculty of Agriculture, Department of Horticulture, Yozgat, Türkiye.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Today, changing lifestyles and conscious consumer behavior force a quality increase and improvements in food. Conscious consumers act sensitively about healthy living and tend to natural products that both protect against diseases and meet their nutritional needs. Some compounds contained in fruits and vegetables are known to have positive effects on human health. Grapes are among these fruits with their high nutritional value and high content of antioxidant compounds, especially phenolic compounds. Therefore, the use of different grape extracts in areas such as food, health, and cosmetics has become popular in recent years. Different methods can be employed to preserve this composition in products with rich content, to minimize extraction losses, as well as to enable the plant to synthesize these components in higher amounts without converting them into extracts. Endogenous hormones are known to be particularly effective in the production of these components by activating different signaling mechanisms. In this study, the potential of using methyl jasmonate as an elicitor in Merzifon Karası grape, a colorful grape variety, was evaluated. Grapes were sprayed once (once application) immediately after veraison, twice (twice applications) immediately after veraison, and 15 days after veraison (doses of 0; 1; 2.5, and 5 mM). At harvest, the clusters were harvested and anthocyanin, phenolic compounds (spectrophotometrically and by HPLC), amino acid and organic acid contents of grape berries, and fatty acid contents of grape seeds were determined. The results showed that methyl jasmonate as an elicitor was highly effective in enriching the berry composition of grapes.
Keywords: Grape, Elicitor, Jasmonic acid, Secondary metabolites
Cite this paper: Emine Sema Çetin, Ali Tufan Erşenel, Enrichment of Extract Composition of Merzifon Karası Grape Cultivar with MeJA Elicitor, International Journal of Plant Research, Vol. 13 No. 1, 2023, pp. 43-51. doi: 10.5923/j.plant.20231301.03.
Article Outline
1. Introduction
- Grapes have a special position because of the many valuable components in their composition. Besides its rich content of vitamins and minerals, grapes are known to have approximately 1600 compounds derived from phenylpropanoids, isoprenoids, and alkaloids that are effective in many diseases. With the effect of these compounds, grapes are known to prevent cancer formation, bad cholesterol, high blood pressure, heart attack risk, Alzheimer's, Parkinson's, dementia, and many other neurodegenerative diseases [1].The synthesis of these components depends largely on their response to environmental conditions and adaptation to the environment. Endogenous hormones are also effective in the formation of this response. As it is known, hormones are organic compounds that have extremely important roles in plant growth and development, can be transported within the plant, and can show their effects even in very small doses. Jasmonic acid (JA) is one of the plant hormones that play an important role in plant development and many physiological and biochemical processes [2]. It was first isolated in 1962 from the oil extracted from the jasmine (Jasminum grandiflorum) plant [3]. JA is known to be effective in activating the natural defense mechanism in plants, stimulating abscission and closure of stomata, and promoting chlorophyll degradation, respiration, ethylene, and protein synthesis [4-6]. In all these processes, it promotes increased secondary metabolite synthesis [7,8]. Methyl jasmonate (MeJA) is an odorous volatile compound that is the methyl ester of JA. It is known that studies on JA or MeJA are mostly conducted to enrich phenolic compounds in the presence or absence of stress. In one of the studies in this field, Larronde et al. [9] treated clusters of Cabernet Sauvignon grape variety with MeJA. As a result of the study, it was found that MeJA application promoted the accumulation of piceid in leaves and trans-resveratrol in berries. In another study conducted on the same variety, MeJA application was applied and it was determined that trans-resveratrol, piceid, ɛ-viniferin, δ-viniferin and pterostilbenes increased compared to the control [10]. Vezzulli et al. [11] also applied MeJA to the clusters of Barbera grape variety. As a result of their study, they found that MeJA significantly increased the production of trans-resveratrol and ε-viniferin in the berries during the ripening period. In another study, Ruiz Garcia et al. [12] applied MeJA at a dose of 10 mM to the Mourvedre grape variety, starting just before the veraison period, 3 times at 3-day intervals. They reported that the amounts of anthocyanins and proanthocyanins in the treated clusters increased significantly compared to the control. In a study conducted in Tempranillo, one of the important wine grape varieties, it was determined that pre-harvest application of MeJA to the vines significantly increased the total anthocyanin content [13]. Wang et al. [14] examined the effect of MeJA on chlorophyll, vitamin C, soluble protein, sugar, nitrate, total phenols, flavonoids, volatile components, enzymatically produced pyruvic acid content and antioxidant activity and stated that MeJA was very effective in increasing quality components. In this study, the effects of MeJA applications on the biochemical contents of grapes were examined. Exogenous MeJA applications were made in Merzifon Karası grape variety and the amounts of phenolic compounds, total phenolic matter, total flavonols, total flavanols, and anthocyanins, as well as amino acids and organic acids, and the amounts of fatty acids (in seeds) which are also very important in human health were examined. It was aimed to reveal the potential of MeJA on these components. The composition of the berry was analyzed with its many components, and the potential for conversion into a rich product of extracts will increase in further studies with the increase in the amounts of compounds effective in food, cosmetics, and health in light of the data obtained.
2. Material and Methods
2.1. Material
- Merzifon Karası grape variety grafted on 1103 Paulsen (1103 P) American grapevine rootstock was used as material in the study. Merzifon Karası grape variety is one of the wine grape varieties with thin skin, and round and medium-sized berries. The berry skin color is blue and black and it has medium-sized and dense clusters. The treatments were carried out in a 12-year-old vineyard established in the Merzifon District of Amasya Province (Figure 1). MeJA was 99% pure and commercially available (CAS no: 39924-52-2, Sigma Aldrich, Darmstadt, Germany). It was prepared by dissolving in ethyl alcohol and diluted to 1 mM, 2.5 mM, and 5 mM. Deionized water containing equal amounts of alcohol was used as a control.
 | Figure 1. A view from the vineyard |
2.2. Methods
- MeJA treatments were applied in two separate application periods as one period and two periods, taking into account the veraison period. The once application (OA) was made once on August 2, 2021, immediately after the veraison period. The twice application (TA) was made twice, the first on the same date and the second two weeks after the first application (August 16). At harvest, clusters were harvested (Figure 2) and analyzed as detailed below.
 | Figure 2. A view of ripe grape |
 | Figure 3. Samples in total flavonol analysis |
 | Figure 4. Samples in anthocyanin analysis |
3. Results and Discussion
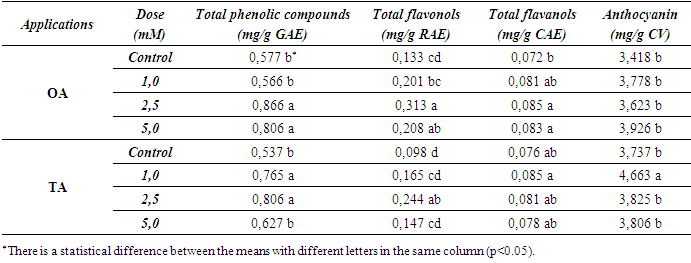
- The effects of MeJA treatments on berry composition were examined and the results are detailed below. In terms of total phenolic compounds calculated as gallic acid eq, the highest values were found in the treated grapes (Table 1).
|
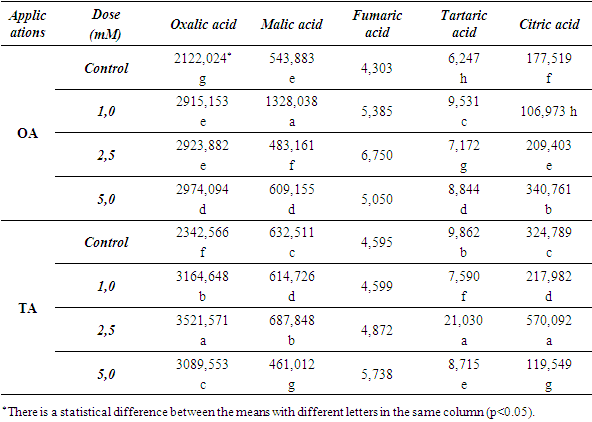
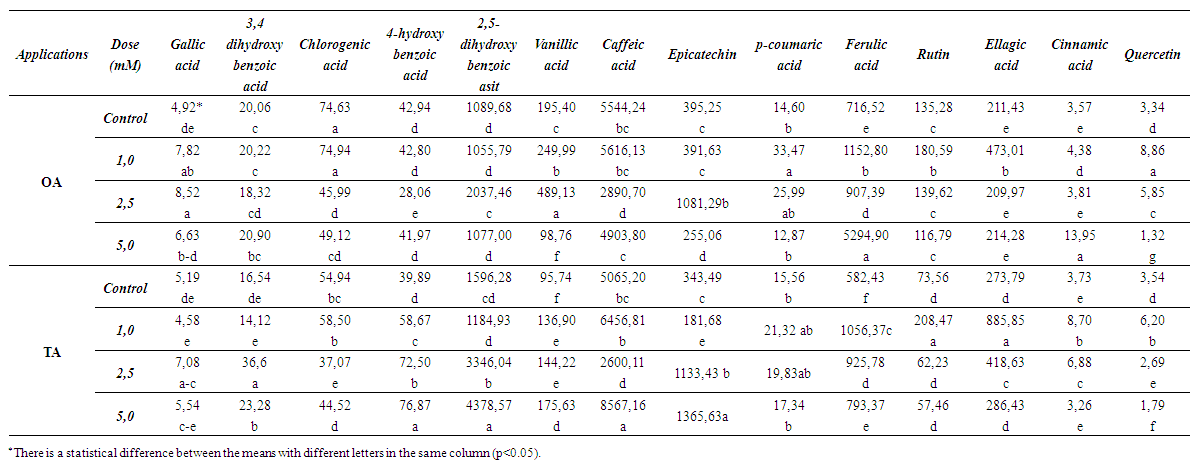 | Table 2. Changes in the amounts of some phenolic compounds (ppm) |
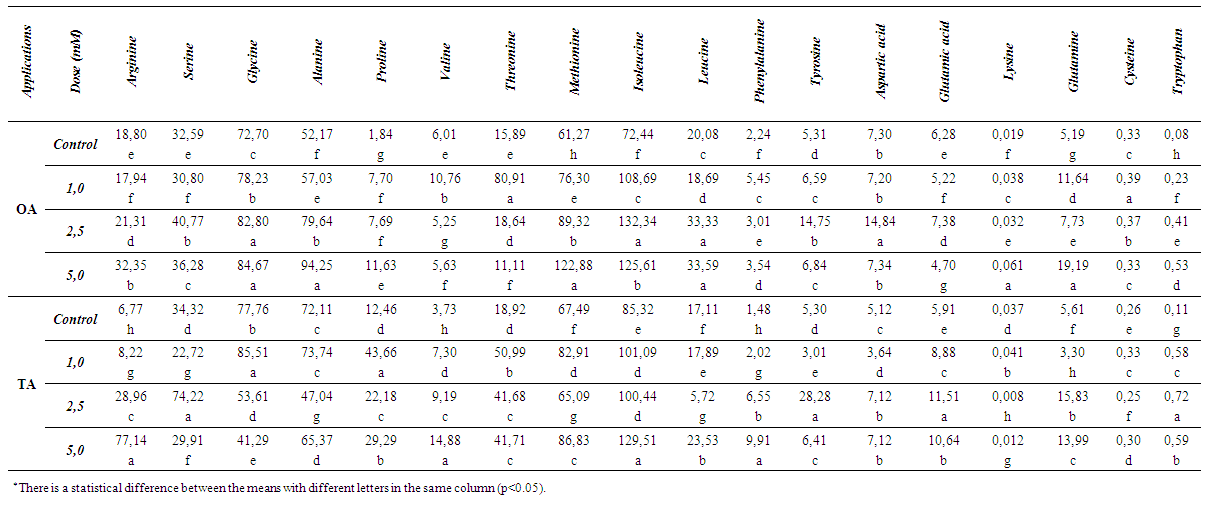 | Table 3. Changes in the amounts of amino acids (ppm) |
|
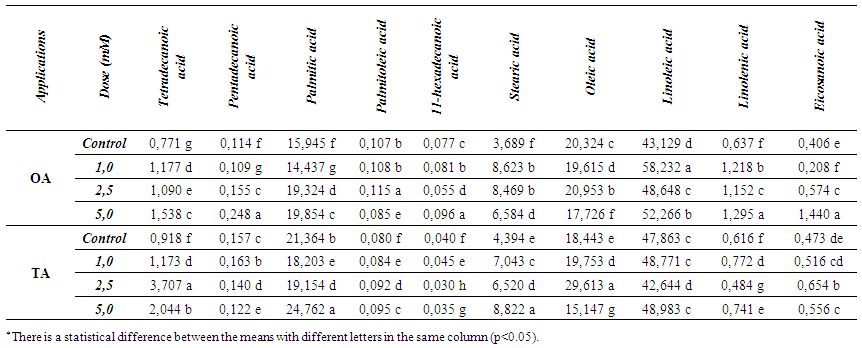 | Table 5. Changes in the amounts of fatty acids (%) |
4. Conclusions
- It is known that plant extracts in particular have a significant consumption as food and nutritional supplements. Studies aiming to increase the components in the main product from which they are obtained in order to further enrich the content of extracts, which are rich in nutrients and components that have a significant effect on human health, are also extremely important. It is also known that many studies have been carried out on different plants in this field. Especially in plant materials that are already known to be rich in these components, these studies will make the final product composition more valuable by tolerating the losses in the extract process. This study showed that MeJA applications had positive effects on all parameters examined. It is possible to transfer this application to practice by taking the period and doses used to increase the amount of the desired target compound in studies in this field as a reference. However, it is also thought that the diversity of applications is limited in the studies on compound enhancement. Comprehensive studies using different pre-applications are needed.
ACKNOWLEDGEMENTS
- Author Contribution: Emine Sema CETIN contributed to this work in the experimental design and setup, lab processing of samples, data analysis, manuscript writing and discussion. Ali Tufan ERSENEL contributed to lab processing of samples, data interpretation, manuscript writing and discussion. Authors read and approved the final manuscript.Conflict of interest: E.S. Çetin and A.T. Ersenel declare that they have no competing interests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML