-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2022; 12(1): 8-19
doi:10.5923/j.plant.20221201.02
Received: Oct. 15, 2020; Accepted: Nov. 16, 2020; Published: Aug. 30, 2022

Yield Performance of Wheat Genotypes (Triticum aestivum L.) and Occurrence of Native Arbuscular Mycorrhiza Fungi in Contrasted Conditions of the Bimodal Humid Forest Zone of Cameroon
Charly Emmanuel Mam1, Eddy Leonard Mangaptche Ngonkeu1, 2, Gabriel Mahbou Somo Toukam1, Fanche Aminatou Mongoue1, 2, Mmala Patrick Tsimi1, Honore Tekeu1, Arielle Wada1, Bolomigui Boyomo1, Julie Doriane Kamko1, Arlette Foko1, Adrienne Ngo Ngom1, Jeanne Sidonie Minda1
1Department of Plant Biology, Faculty of Science, University of Yaoundé I, Yaoundé, Cameroon
2Institute of Agricultural Research for Development, Nkolbisson, Yaoundé, Cameroon
Correspondence to: Charly Emmanuel Mam, Department of Plant Biology, Faculty of Science, University of Yaoundé I, Yaoundé, Cameroon.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Increased temperature and degradation of soil fertility are the principal constraints affecting wheat production in Cameroon. As such, this study aimed to determine the potential of native Arbuscular Mycorrhiza (AM) fungi in yield performance and variability of wheat genotypes cultivated in contrasted conditions of the bimodal humid forest zone of Cameroon. For this, 34 wheat genotypes were sown following an incomplete alpha-lattice design. The genotypes were evaluated on the basis of their yield potential (grain weight) and their affinity to mycorrhiza symbiosis. The efficiency of the occurred mycorrhiza was verified on two wheat genotypes and compared to the standard chemical fertilization used and a control test. Wheat grain weight, shoot dry mass, number of leaf, shoot length and leaf area were collected for this purpose. Biplot analysis revealed positive significant correlations (r=0.83 P<0.01; r=0.77, P<0.01) between the grain weight of wheat genotypes and mycorrhiza parameters (percentage of AM colonization and number of AM spores respectively) in Mbankolo (high altitude) and positive significant correlations (r=0.56, P<0.01; r=0.57, P<0.01) between the same parameters in Nkolbisson (low altitude). The grain weight and mycorrhiza parameters explained 90.1% and 71.3% of variance among all the wheat genotypes in high and low altitudes respectively. The following genotypes SST015, SST087, SST866 and SST88 in high altitude and the following genotypes Nd643-5, Kenya2, Babax8 and SST895 in low altitude were identified to have the highest mycorrhiza symbiotic affinity and yield performances. The mycorrhiza spores isolation and morphological identification revealed the presence of three species in wheat rhizosphere in both study sites: Scutellospora sp, Gigaspora sp, and Septoglomus sp. The species Scutellospora sp, was identified to be present and dominant in both study sites. These latter were observed to have a high growth and yield enhancing ability on wheat varieties compared to the conventional chemical method of fertilization hence could be use as efficient bio-fertilizer. Nonetheless, it will be necessary to perform molecular phylogeny to identify each AM specie in order to individually evaluate their efficiency on wheat growth and yield. Also, the targeted wheat genotypes can be used to promote large scale production in both sites and vulgarize to local farmers. Additionally, the determination of the mechanism underpinning their symbiotic preferences will be essential in targeting the genes implicated to be used for breeding purposes.
Keywords: Bio-fertilizer, Wheat symbiotic affinity, Genotype variability, Yield
Cite this paper: Charly Emmanuel Mam, Eddy Leonard Mangaptche Ngonkeu, Gabriel Mahbou Somo Toukam, Fanche Aminatou Mongoue, Mmala Patrick Tsimi, Honore Tekeu, Arielle Wada, Bolomigui Boyomo, Julie Doriane Kamko, Arlette Foko, Adrienne Ngo Ngom, Jeanne Sidonie Minda, Yield Performance of Wheat Genotypes (Triticum aestivum L.) and Occurrence of Native Arbuscular Mycorrhiza Fungi in Contrasted Conditions of the Bimodal Humid Forest Zone of Cameroon, International Journal of Plant Research, Vol. 12 No. 1, 2022, pp. 8-19. doi: 10.5923/j.plant.20221201.02.
Article Outline
1. Introduction
- Wheat is the second most consume cereal after maize and rice with a world annual production of approximately 766 million ton (FAO, 2019). Wheat represents one of the main sources of food security in Africa (Negassa et al., 2013). In Cameroon, its demand has increase along the years with an increase in wheat consumption of 98% in urban strata and 90-91% in non-urban strata (Engle-Stone and Brown, 2015). However, 100% of the total domestic consumption is satisfied by import translating Cameroon vulnerability to food security risk (PAM, 2011). In Cameroon, the production which is almost non-existent is nonetheless estimated at 900 tons annually (FAO, 2019), Highly inferior to the national importation estimated at 725,000 tons in 2015 (Mocauley et Ramadjita, 2015). To satisfy this population demand, the government allocated an amount of 103 billion of FCFA to import approximately 518, 000 tons in 2012 (Yamdjeu, 2012). Facing this situation, the Cameroon government is urge to implement appropriate strategies to reduce the wheat dependency, to minimize price fluctuation and attain a minimum threshold for self-sufficiency for this commodity.Increase temperature has been the major constraint responsible for 58-92% decrease in wheat yield in this sub region. Evaluations to adaptation and breeding programs have been conducted by local researchers to obviate this constraint (Ayuk-takem, 1983). However, these trials have seriously decreased the soil productivity capacity along the years due to the use of conventional practices. An alternative solution might be the application of natural method of fertilization, which will maintain at the same time a good soil health and enhance tolerance of wheat plant to high temperature. But none of such has yet been done in Cameroon.Arbuscular mycorrhizae also called endomycorrhizae are the most ancient, widespread and common soil denizens, that establish an association with the root of 80% of vascular plant species (Wang and Qiu, 2006). Arbuscular Mycorrhizal (AM) fungi are endophytes that constitute the phylum of Glomeromycota. They are ubiquitous in soil around the globe and have been associated with improve plant growth for over 100 years (Hamel, 1996; Singh and Adholeya, 2013). These symbiotic fungi are the main components of the soil micro-biota in most of the agroecosystems and account for 25% of the biomass of soil micro flora and micro fauna combined (Singh and Adholeya, 2013). Mycorrhizae are mutualistic symbiotic associations based on bidirectional nutrients transfer between soil fungi and the roots of vascular plants, where the plant supplies the fungi with sugars produced by photosynthesis, while the hyphae network improves the plant capacity to absorb water and nutrients (Roy-Bolduc and Hijri, 2011). AM fungi are obligate biotrophs since the rely on their host plant to proliferate and survive (Pozo and Azcón-Aguilar, 2007). They are also known to provide several ecosystem services including promoting plant growth, nutrient uptake and enhancing soil stability (Velázquez et al., 2016), improvement of water retention through mycorrhizal symbiosis (Bernardo et al., 2019) and confer to crops resistance to biotic and abiotic stress (Augé and Moore, 2005; (Filho et al., 2017).This enhancing ability of Arbuscular Mycorrhizal fungi on plant growth and adaptation is generally determined by the degree of symbiotic compatibility, which is affected by the host plant and fungi species (Silva et al., 2018). This evidence was also proven by (Munkvold et al., 2004; Stover et al., 2012; Monier et al., 2017), who emphasized on the large functional variability among distinct AM fungi species and fungi isolates of the same species. Hence the continuous study of Arbuscular mycorrhizal diversity is a necessity in order to target the predominant species and their corresponding host. This has justify the extensive studies of AM fungi diversity in natural ecosystems. However, few studies have been conducted in order to appreciate the diversity and influence of these indigenous mycorrhizal fungi in plant growth and yield in agricultural lands (Singh and Adholeya, 2013; Loit et al., 2018). Indeed, agricultural lands are constantly subjected to human intervention which tends generally to reduce AM fungi diversity, their colonization ability thereby reducing their impact in crop adaptation and yield (Singh and Adholeya, 2013; Loit et al., 2018). Therefore, there is an urgent need in exploring these indigenous mycorrhiza diversities, their influences in increasing crops adaptations and yield, which might be highly informative on their presence, host specificity and symbiotic compatibility with most of the cultivated crops in agricultural lands in view of promoting good fertilization processes.In Cameroon, Kamko et al. (2020) showed that Gigaspora margarita is an efficient mycorrhiza species, which can be used as an effective bio-fertilizer for the domestic propagation of Prunus africana. Mbogne et al. (2015), studied the diversity of AM fungi of pumpkins, and isolated two AM fungi species Glomus and Acaulospora, with Acaulospora identified to be the abundant species. Nzweundji et al. (2015), identified three families associated to Prunus africana, which included Gigasporaceae (Gigaspora margarita), Acaulosporaceae (Acaulospora tuberculate, A. longula and Enthrophosphora colombiana) and Glomeraceae (Glomus manihotis and G. etunicatum). Bechem and Alexander, (2012), showed that Gnetum Spp. had a high preference to ectomycorrhizal colonization of genus Scleroderma (Scleroderma sinnamariense), which may highly contribute to domestication efforts. Therefore, undergoing this same purpose in contrasted wheat cultivated areas might be highly informative in highlighting not only the mycorrhizal diversity and their influence in wheat yield capacity but also inform of the degree of wheat symbiotic need in constraint environments. Since plant mostly adhere to symbiotic association when facing high level of abiotic stress. This study might be a promising step for the restoration of soil productivity capacity in wheat agricultural areas and also for the improvement of wheat productivity in sub-Saharan Africa and Cameroon in particular. Furthermore, a recent report made by (Sawers et al., 2008), mention the fact that, in a study comparing the performance of wheat varieties developed before and after 1900, varieties developed before 1900 were more responsive to AM colonization than those developed later and it has been suggested that plant breeding has selected against AM association. However, in their report, it was mention that the observed differences in responsiveness were largely determined by an increased ability of modern lines to take up phosphate without AM fungi (i.e. a reduction in dependence, not a loss of compatibility with the fungus). This signifies cultivating wheat with a high level of chemical input. But high level of inputs decreases soil fertility along the years. Thus investigating the influence of mycorrhizal symbiosis on wheat yield and symbiotic preference in cultivated land will be a promising introduction to encourage plant breeders to put in place new wheat lines with increase in dependency to AM colonization. This justifies the aim of this study, whose purpose was to determine the potential of native Arbuscular Mycorrhiza (AM) fungi in yield performance and variability of wheat genotypes cultivated in contrasted conditions of the bimodal humid forest zone of Cameroon.
2. Materials and Methods
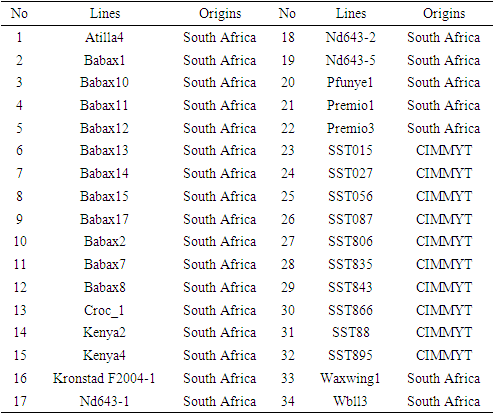
- Field experiment The experiment was conducted on two sites of different altitudes of the bimodal humid forest zone of Cameroon. The high altitude site of 1057m above the sea level, with average temperature and rainfall of 17°C and 1592.2 mm respectively, was located in Mbankolo mountainous zone. This site had a relative humidity of 77.53%. The low altitude site standing at 650m above the sea level with average temperature and rainfall of 23.5°C and 1560 mm respectively was located in lowland of Nkolbisson zone, with a relative humidity of 62%. A total of thirty-four (34) wheat genotypes were evaluated during a period of March-August 2016. Accessions were collected in Africa, Mexico and some from international Maize and wheat improvement center (CIMMYT) (Table.1). These genotypes were evaluated in an incomplete alpha-lattice design with 3 repetitions. The wheat genotypes were scored on the basis of the grain weight (Wgr), which was collected after harvest using a four digits’ unit weight balance (KERN, Germany).
|
3. Results
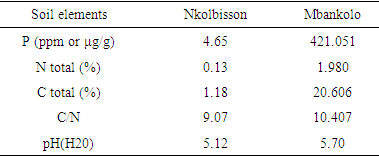
- Soil characterization of both study sitesThe soil analysis presented different characteristics on both experimental study sites (Table.2). In Nkolbisson (low altitude) study site, the soil had a pH value of 5.12, whereas in Mbankolo (high altitude) study site the soil had a pH value of 5.7 (Table.2). Nkolbisson presented low mean value of available phosphorus (4.65μg/g) compared to Mbankolo (421.051μg/g). The available nitrogen and carbon ratio (C/N) was also evaluated for both sites and the ratio of 9.07 was obtained at Nkolbisson compared to a C/N ratio value of 10.407 obtained at Mbankolo (Table.2).
|
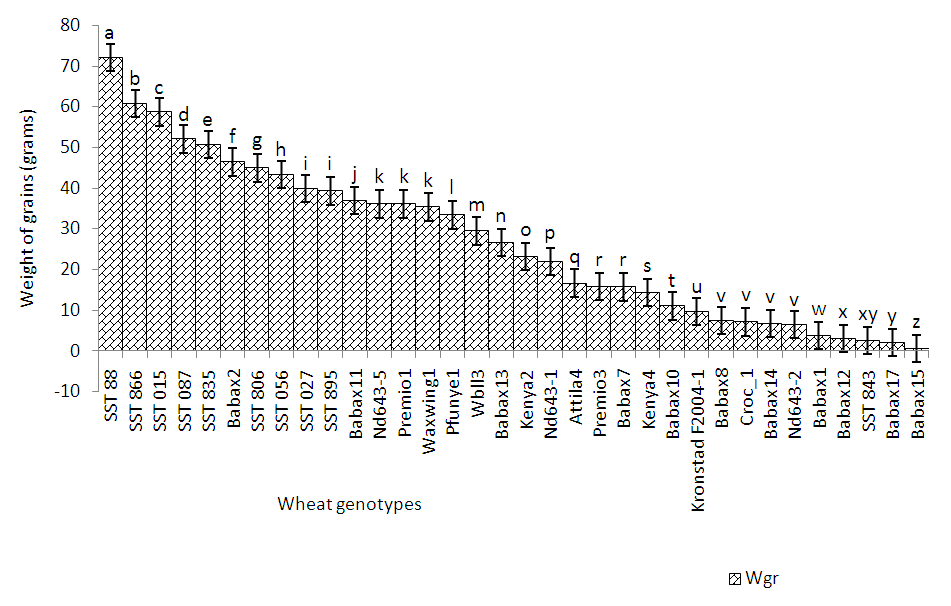 | Figure 2. Yield performance of wheat genotypes in Mbankolo |
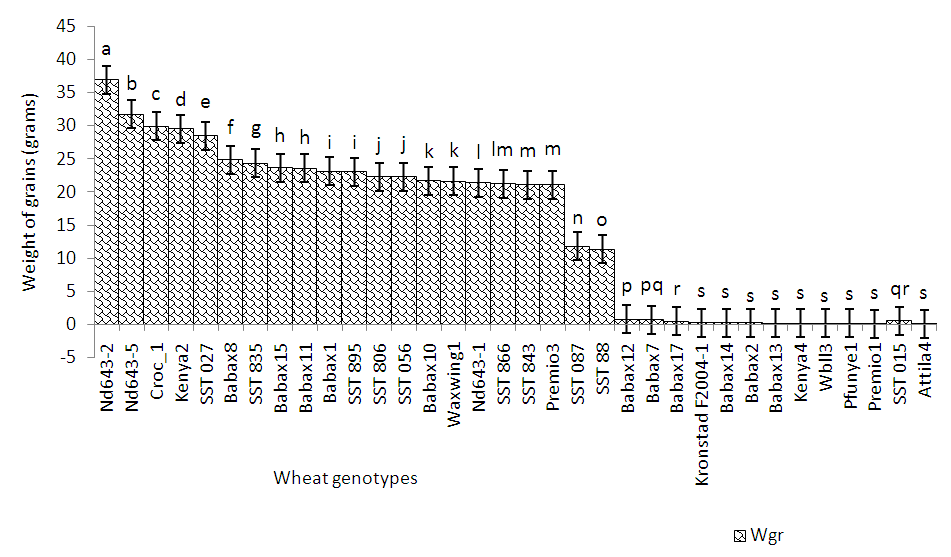 | Figure 4. Yield performance of wheat genotypes in Nkolbisson |
|
|
|
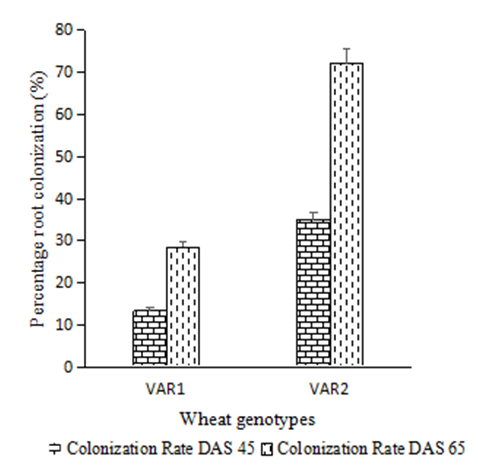 | Figure 6. Root percentage colonization of wheat genotypes |
4. Discussion
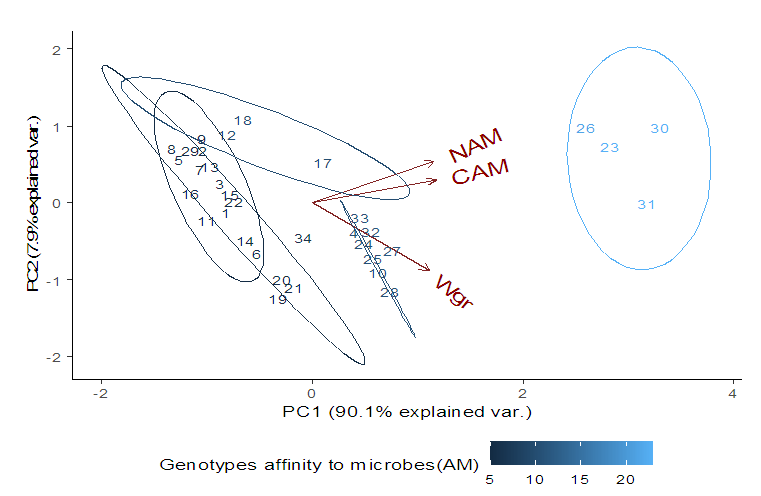
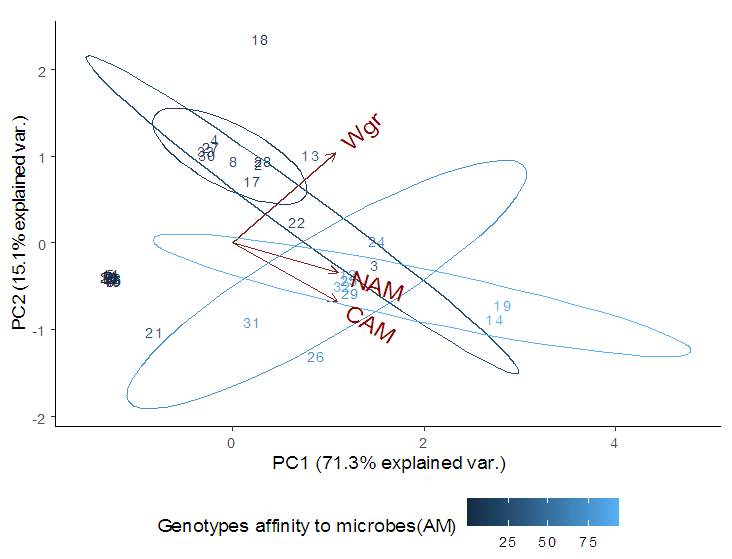
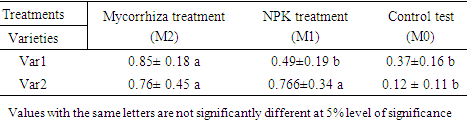
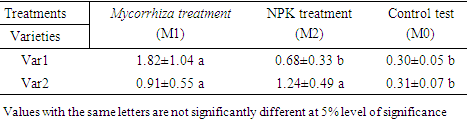
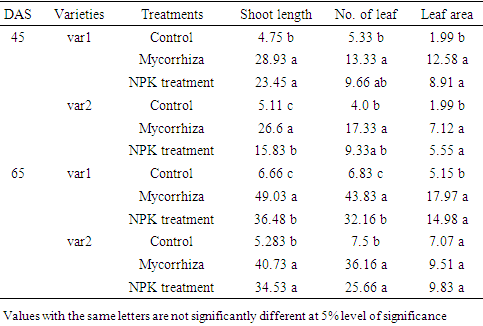
- Soil characteristics of both study sitesThe contrasted soil characteristics observed in both study sites, was due to the difference in altitude, since according to the result obtained by (Saeed et al., 2014), the soil physico-chemical properties showed a positive correlation with elevation gradient (altitude). In Nkolbisson (low altitude), the soil pH was 5.12, lower than the critical soil pH (5.29) for wheat preconized by (Baquy et al., 2016). Whereas in Mbankolo (high altitude), the soil pH (5.7) was higher than the critical and tolerable soil pH, but lower than the adequate pH (6.0-7.0) for wheat production (Brann et al., 2009). Phosphorus content in low altitude was observed to be lower compared to high altitude and this can be explained by the low soil pH that affected the availability of important element in the soil. A similar result was observed in the same site by Tekeu et al. (2015).Relation between occurred Abuscular mycorrhizal fungi, yield and wheat genotypesPositive significant correlations revealed between the grain weight, number of AM spores and percentage colonization in Mbankolo (high altitude) and Nkolbisson (low altitude), indicate a positive influence of mycorrhizal symbiosis on yield performance of wheat. A similar result was observed by (Essiane-Ondo et al., 2019), in a study of wheat landraces with low mycorrhizing ability at field respond differently to inoculation with introduced or indigenous arbuscular mycorrhizal fungal communities. It was observed in this study that the community of arbuscular mycorrhizal fungi can have an effect on yield, even in wheat landraces with low mycorrhizogenous ability. This also justifies the positive influence of the few AM species identified in both study sites on wheat grain weight. Nonetheless, a high correlation was observed between mycorrhiza parameters and wheat grain weight in Mbankolo (r= 0.83 and r= 0.78) compared to Nkolbisson (r= 0.55 and r = 0.56). This result could be justified by the observations made by (Cardoso Filho, Sobrinho, & Pascholati, 2017; Francis and Read, 1995), where they mention a spectrum of fungal impacts in which some species respond mutualistically, while others, putative host or nonhosts, are antagonized or antagonized the action of other species. Since three species were identified in Nkolbisson and the efficiency of a particular species of mycorrhiza might sometimes be influenced by the presence of another mycorrhizal species that is not specific to the host. It was also observed that AM parameters highly contributed to the variation in wheat genotypes in both study sites, as these parameters were highly loaded in PC1 that respectively explained 90.1% and 71.3% of variance in Mbankolo (high altitude) and Nkolbisson (low altitude) respectively. This result justifies the allegation made by (Silva et al., 2018) mentioning the fact that the enhancing ability of arbuscular Mycorrhizal fungi species on plant growth and adaptation is generally determined by the degree of symbiotic compatibility, which is affected by the host plant and fungi genotypes. Hence the symbiotic preference of the wheat genotypes might have affected the mycorrhiza parameters and thus has caused this variation. Increase number of genotypes (Nd643-5, Kenya2, Babax8, Croc-1, SST027, SST056, SST086, SST843 and SST88) showed high affinity to mycorrhiza colonization in Nkolbisson compared to Mbankolo (SST015, SST087, SST866 and SST88). This result could be justified by the low soil fertility observed in Nkolbisson site compared to Mbankolo characterized by reduced available phosphorus, which is the principal element mycorrhiza supply to plants. Hence increasing their mycorrhiza affinity permitted them to easily acquire essential elements for their growth and yield. Also, the increase in temperature in Nkolbisson (low altitude) study site might have reduced the water availability in the soil due to increase in evapo-transpiration, hence disturbing the translocation of nutrients from the soil to the plant (Altuhaish et al., 2014). Thus these symbiotic associations in this midst could be extremely beneficial to the wheat plant. These allegations were confirmed through a study conducted by Al-Karaki, McMichael, and Zak, (2004), when working on the field response of wheat to Arbuscular Mycorrhizal symbiosis in drought stress, observed that mycorrhizal symbiosis enhances wheat biomass and yield. Occurred of AMF species in both study sitesMorphological characterization of the occurred AM permitted to identify three Arbuscular Mycorrhizal species that is Septoglomus sp, Gigaspora sp, Scutellospora sp, with dominant species in the rhizosphere of wheat cultivated in both altitudes study field identified to belong to the genus Scutellospora and family of Scutellosporaceae following the revision of Scutellospora and description of five new genera and three new families in the arbuscular mycorrhiza-forming Glomeromycetes (Oehl, de Souza & Sieverding, 2008). A different result was obtained by, Velázquez et al., (2016) who obtained as result a dominant AM fungi community in wheat rhizosphere belonging to the family of Acaulosporacea. This difference in result could be explained by the difference in studied environment and wheat genotypes. A high number of AM spores were present in the rhizosphere of wheat genotypes in Nkolbisson (low altitude) study site, compared to Mbankolo (high altitude) study site. This result indicates the increase in dependency of wheat plant to AM symbiosis in low soil fertility areas (Campos et al., 2018; Rubio et al., 2003), and high temperature areas as mycorrhizal trigger the metabolic response of wheat plant in order to increase their tolerance to drought (Al-Karaki et al., 2004; Bernardo et al., (2019); Llorens et al., 2019).Influence of occurred mycorrhiza on wheat yield and biomass Overall, the mycorrhiza treatment showed a high enhancing ability on wheat grain weight and biomass compared to the other treatments. This enhancing growth ability of mycorrhiza was also observed by (Pérez et al., 2016), in studying the effect of inoculation with Arbuscular Mycorrhizal on selected spring wheat lines, obtained as result an enhancing yield potential of spring wheat by Arbuscular Mycorrhizal symbiosis. On the other hand, the responding ability to mycorrhiza colonization varied in function of the wheat genotype. These results simply corroborate the observation made by (Munkvold et al., 2004; Stover et al., 2012b); Smith et al., 2004; Monier et al., 2017; Silva et al., 2018), mentioning the fact that there exist a variation in the degree of specific symbiotic compatibility of the wheat plant. This might justify the variation in response in yield and growth of the two wheat genotypes.
5. Conclusions
- The purpose of this study was to determine the potential of native Arbuscular Mycorrhiza (AM) fungi in yield performance and variability of wheat genotypes cultivated in contrasted conditions of the bimodal humid forest zone of Cameroon. The results permitted us to conclude that, the following wheat genotypes SST015, SST087, SST866 and SST88 in Mbankolo (high altitude) and the following genotypes Nd643-5, Kenya2 and Babax8 in Nkolbisson (low altitude) had a high yield potential in each of these areas and showed a high symbiotic affinity with AM fungi. Thus determining the mechanism underpinning these symbiotic preferences will be essential in targeting the genes implicated and important for breeding purposes. The Mycorrhiza parameters were observed to highly influence the variability of wheat genotypes in both study sites, hence could be essential criterion for plant breeders in wheat improvement. Scutellospora sp., Gigaspora sp., and Septoglomus sp., were morphologically identified as present in the rhizosphere of wheat lines in both altitudes and were observed to have a high growth and yield enhancing ability on wheat varieties compared to the conventional chemical method of fertilization, hence could be use as efficient bio-fertilizer. Nonetheless, it will be necessary to perform molecular identification (molecular phylogeny) to identify each AM specie in order to individually evaluate their efficiency on wheat growth and yield.
Conflicts of Interest
- The authors declare that there is no conflict of interest regarding the publication of this paper.
Data Availability
- The data analysis used to support the findings of this study and other supplementary images related to this study are available from the corresponding author upon request.
ACKNOWLEDGEMENTS
- A special thanks to the MIC-CERES project (Microbial eco-compatible strategies for improving wheat quality traits and rhizospheric soil sustainability), Agropolis Fondation (grant AF project ID 1301-003), investment d’avenir programme (with reference number ANR-10-LABX-0001-01), Fondazione Cariplo (grant FC project ID 2013-1888), Institute of Agricultural Research for Development (IRAD) and University of Yaoundé I.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML