-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2021; 11(2): 21-31
doi:10.5923/j.plant.20211102.01
Received: Nov. 21, 2021; Accepted: Dec. 3, 2021; Published: Dec. 15, 2021

The Effects of Methyl Jasmonate on Calcium Excess: Changes in Mineral Compounds and Physical-Biochemical Parameters in American Grapevine Rootstocks
Emine Sema Cetin, Selda Daler, Serpil Kizilay
Department of Horticulture, University of Yozgat Bozok, Yozgat, Turkey
Correspondence to: Emine Sema Cetin, Department of Horticulture, University of Yozgat Bozok, Yozgat, Turkey.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The stress situation occurs when plants encounter conditions that are unsuitable for them. Abiotic factors are the most common stressors. In abiotic stresses, imbalances in the nutrient intake by plants have an important share. This situation, which is observed when the intake of some elements is restricted, or conversely, the solubility of some of them increases and becomes toxic, can cause yield and quality losses, and in extreme cases, the death of plants. It is common for high-lime soils to have high pH, thus, nutrient intake problems. This situation brings with it many negatives. Chlorosis is observed in this type of soil, the string intervals of young leaves are yellowing and root development becomes limited. Applications used in soil neutralization, such as leonardite, humic acid, and micronized sulfur, are applications that cannot give results in a short time. It is therefore of great importance that the plant endures in these environments. Today there is a focus on genetic engineering for this purpose. But these methods are expensive, requiring know-how. So finding natural, easy-to-use, practical, and harmless alternatives to human health is at the forefront. Plants are known to synthesize certain hormonal compounds that act as signaling molecules under stress, and therefore some growth regulators are used to provide stress resistance. These signals include jasmonic acids. Jasmonic acid, or its methyl ester, jasmonates, are compounds considered plant hormones, with a multifaceted effect being stimulating, inhibitory, and protective. This research also investigated the effects of exogenous methyl jasmonate applications in three American vine rootstocks (5 BB, 41 B, and 1103 P) that differ in lime sensitivities in lime-containing environments. Cuttings were planted in to polyethylene pots prepared by adding equal volumes of sterile pearlite and turf and placed in the greenhouse for the growing period. They are systematically irrigated with ½MS solution. After two months following planting, rooting, and leafing of the cuttings, calcium oxide (0, 10, 25, and 40% CaO) was applied to root regions. MeJA (150, 300, and 450 ppm) in spray form, was applied to all leaves. A two-month period of improvement was followed after the MeJA application, the trial was terminated. Physical (shoot weight, shoot length, the average number of leaves per shoot, level of damage, rooting rate) and biochemical (chlorophyll amount, degree of membrane injurity, proline amount, total phenolic and mineral compound content) parameters were conducted to measure the level of stress in plants. Data obtained from the study reveal that MeJA has the potential to be used to counter stress caused by lime in American grapevine rootstocks.
Keywords: Grapevine rootstock, Methyl jasmonate, Phenolic compounds, Mineral compounds
Cite this paper: Emine Sema Cetin, Selda Daler, Serpil Kizilay, The Effects of Methyl Jasmonate on Calcium Excess: Changes in Mineral Compounds and Physical-Biochemical Parameters in American Grapevine Rootstocks, International Journal of Plant Research, Vol. 11 No. 2, 2021, pp. 21-31. doi: 10.5923/j.plant.20211102.01.
Article Outline
1. Introduction
- The grapevine is one of the world's economically important fruit species. Because it is less selective than many cultural plants for climate and soil requirements and is one of the oldest agricultural crops of human beings, its cultivation is widely spread across the world. Turkey has a variation potential for vine genes along with the long-established culture of vineyard due to Turkey's location on the climate belt best suited for viticulture, at the intersection of the vine's gene centers [1]. Clay and lime content is known to be high in Turkey territory and organic matter content is generally low. Also, 14.14% of the territory of the Aegean region, 34.21% of the territory of the Mediterranean region, and 37.08% of the territory of the central-south region are very limy. As the amount of lime in the soil increases, deficiencies occur in the intake of other elements such as magnesium, mangan, and zinc, mainly iron. One of the most common nutrient deficiencies in viticulture is the lack of iron in soils with a lime content of more than 20% [2]. Hence, the iron deficiency is not only due to the inadequate amounts of iron in the soil, but also because it is not in useful form for plants. However, given that 26.87% of Turkey's territory is below 4.5 mg/kg, which is considered critical in terms of useful iron, iron deficiency in vineyards is inevitable. In case of deficiency, the string intervals of young leaves are yellowing, and the strings remain green [3]. Root development is also limited in these conditions, and this adversely affects the quality and yield of the plant. The vine plant is actually a plant that can be produced easily and successfully with cutting. But it is imperative to use American vine rootstocks for economic viticulture due to the Phylloxera pest. Hence, in the case of nutrient intake, the nutrition of the rootstock comes to the fore. The durability of the grapevine rootstocks varies between them but lags behind the varieties of the V.vinifera species.Applications such as leonardite, humic acid, and micronized sulfur, which are used to neutralize limy soils, are applications that cannot give results in a short period of time, and it is of great importance to increase the resistance of the plant. It has been determined in recent years that the biotic and abiotic stress environments that plants encounter can be mitigated by offering biological approaches, or it can be given the plant durability in this way.Plants are known to transmit a number of signals in their sensitivity processes to stressors. These signals include salicylic acid [4] ethylene [5], and jasmonates [6]. These signals also play a role in initiating defense mechanisms. It is therefore known that some growth regulators can be used to provide resistance to the stress environment [7,8].Jasmonic acid (JA), which is a phytoalexin and forms the plant's active defense mechanism, is a compound [9] that is first isolated from the jasmine (Jasminum grandifolium) plant in the Oxylipids class. JA and its methyl ester, methyl jasmonate (MeJA) [10], are found in all higher plants [11]. Jasmonates are compounds that have multifaceted effects, including stimulating, inhibitory and protective effects, and are therefore considered by many scientists to be in the plant growth regulators class [12]. It is known in general that jasmonates promote root formation in plants [13], block enzymes that cause denaturation of proteins in injuries, are effective in signal molecules and increase β-Carotene synthesis [14], promote germination in seeds [15], are effective in secondary metabolism, affecting genes that regulate the formation of cell wall along with defensive proteins [16]. It is seen that the studies carried out on JA so far have focused on examining the effects on the yield and quality of plants, primarily on stress physiology. It has been stated that carbonic anhydrase activity related to the mechanism of defense against osmotic stress increases with JA [17], that JA improves photosynthetic performance in salt stress in barley [18], and that MeJA in strawberries increases the resistance against water stress [19]. MeJA was determined to delay flowering in long-day plants, inhibit proteinase enzyme activity, increase aromatic components and anthocyanins, and inhibit disease and harmful development [20]. Studies of JA or MeJA in the vine plant are usually intended to increase the quantities of secondary components or to determine the effect on certain diseases. Böttcher et al. [21] conducted a study to determine the effect of the plant hormone JA on grain development and maturation. Krisa et al. [22] stated that the addition of MeJA to Gamay and Cabernet Sauvignon cell cultures increased total piseids. Decendit et al. [23] found that the addition of MeJA to the medium of Gamay cell cultures led to increased flavanols and complex stilbene derivatives. It has been determined by studies that the synthesis of anthocyanin is increased with JA application in Gamay cell cultures [24], again, MeJA application in grapes promotes the accumulation of resveratrol [25], MeJA in the Negramaro variety is effective in increasing the amount of stilbenes [26]. Oçkun [27] indicated that JA and salicylic acid are effective in the formation of the callus in the vine. Malabarba et al. [28] studied the role of jasmonates in tendril movement in the vine and determined that even without mechanical stimulants, jasmonates showed strong activity in triggering tendril attachment. A study conducted in cell suspension cultures in vine also found that MeJA, administered at different doses, promotes tocopherol production [29]. Apart from this, there are studies to determine the effects of JA and MeJA applications against biotic stressors in the vine. One of these studies investigated the role of JA in gaining resistance to P. viticola. The research indicated that gene expressions associated with the JA and SA signaling pathways increased in the early hours after P. viticola vaccination, hence JA was effective in gaining the defense status against the biotrophic pathogen [30]. It is also known that the level of JA increases rapidly after infection in resistant cultivars [31,32], while this increase is limited in susceptible cultivars. Fang et al. [33] conducted a study on Vitis amurensis and stated that drought tolerance can be increased by regulating JA synthesis. In the study of Gülbasar Kandilli et al. [34], the vines were inoculated with Powdery mildew and Downey mildew, SA, JA, and abscisic acid. They were found to be in high quantities shortly after inoculation with pathogens, especially in resistant varieties. In short, JA or MeJA is prominent in studies related to stress.This research is intended to determine how MeJA, a jasmonic acid ester in vine plants grown in lime-containing environments that creates stress in the plant, affects mineral intake, and thus produces many negative consequences. Three American grapevine rootstocks with different sensitivity to lime were selected and the effects of MeJA applications on rootstocks in environments containing different levels of lime were investigated.
2. Material and Methods
- The research was carried out between 2019-2020 in Yozgat Bozok University, Faculty of Agriculture, Department of Horticulture.
2.1. Material
- The research used Kober 5 BB (5 BB), 41 B M.G. (41 B), and 1103 Paulsen (1103 P) American vine rootstock materials, which were obtained from Bursa Agriculture Inc (Bursa/TURKEY).5 BB: It is a hybrid of berlandieri x riparia. It is a strong rootstock and can fit moist and clay soil. It rests well on around 20% active lime, not liking very arid soils.41 B: It is a vinifera x american hybrid. With a short vegetative period, the rootstock has an excess resistance to lime and is especially used for extremely chalky soils.1103 P: It is berlandieri x rupestris hybrid. The rootstock, which develops vigorously and adapts well to clayey-lime soils, is resistant to active lime up to 17-18%.
2.2. Methods
- The preparation of cultivation environment, planting, and applications of lime, and MeJA: The research was planned according to the randomized block design with 3 repetitions and there were 10 plants per repetition. Cultivation environments were prepared by adding equal volumes of sterile pearlite and turf to pots made primarily of polyethylene material. American vine rootstocks were planted in these environments and placed in the greenhouse for the growing period. They are systematically irrigated with ½MS solution to meet water and nutrient needs in these environments. After two months following planting, rooting, and leafing of the cuttings, lime was applied to root regions, supplying 0, 10, 25, and 40% calcium oxide (CaO). Then the rootstocks were divided into groups, and the MeJA, crafted as 0 (control), 150, 300, and 450 ppm in spray form, was applied to all leaves. The control application was realized with water. A two-month period of improvement was followed after the MeJA application to rootstocks in environments with different lime contents, then the trial was terminated and measurements, observations, and analyses regarding physical and biochemical changes were made detailed below.Physical Analyses- Shoot weight: The shoot weight was measured with the help of analytical scales of 0.0001g precision in g.- Shoot length: The shoot length was measured with the help of a ruler in cm.- Average number of leaves per shoot (ANLPS): All leaves on shoots were counted and determined.- Rooting rate: The rooting rate was determined by the ratio of root-forming rootstocks to total rootstocks.- Level of damage: The scale method created by Martinez Barroso and Alvarez [35] was modified and used. Plants that do not have chlorotic tissues resulting from the damage are "level 0"; light yellowing at the leaf edges are "level 1"; yellowings at more than 50% of the leaf are "level 2"; and chloroses which cause the death of the plant are described as 'level 3' damages. Biochemical Analyses- Chlorophyll content: Chlorophyll analyses are determined by Chlorophyll Meter in SPAD.- Degree of membrane injurity: It was determined by measuring the excess electrolyte delivered to the outside from plant cells under stress conditions [36]. Membrane injurity index was calculated in percentage according to the formula below.MII = (Lt-Lc/1-Lc) x 100Lt: EC value before autoclaving/EC value after autoclaving of treatment leafLc: EC value before autoclaving/EC value after autoclaving of the control leaf- Proline content: Determining the proline content in samples was made according to the method of Bates et al. [37]. Proline concentration was determined as μmol/g proline (fresh weight).- Total phenolic content analysis: Extraction was performed according to the method of Kiselev et al. [38]. Total phenolic content analyses were based on Singleton and Rossi [39] using the Folin Ciocalteu colorimetric method. Spectrophotometer readings were performed at a wavelength of 765 nm. Total contents of phenolic compounds were given as mg/g (gallic acid equivalent (GAE)).- Determining the mineral compound content:In the research, phosphorus, potassium, calcium, magnesium, and iron were determined by the Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES) device (Perkin Elmer Otima-8000). The plant samples were burned on the Milestone Start D device [40]. The two-stage temperature program for the burning process was performed. The operation conditions of the device are as follows; Rf power (W) 1450; Injector: Alumina 2 mm i.d.; Sample tubing: Standard 0.76 mm i.d; Drain tubing: Standard 1.14 mm i.d.; Quartz torch: Single slot; Sample capillary: PTFE 1 mm i.d.; Sample vials: Polypropylene; Source equilibrium delay: 15 sec; Plasma viewing: Axial; Processing mode: Peak area; Gases: Argon and Nitrogen; Shear Gas: Air. Wavelength of mineral compounds are as follows; phosphorus: 214,9; potassium: 766,4; calcium: 315,8; magnesium 279,0; iron: 238. Results were given as ppm.
2.3. Statistical Analyses
- The data obtained as a result of the analyses in the research were tested using the SPSS 20.0 package software. The Duncan multiple comparison test was used to determine the differences between group averages, and the numerical values were interpreted accordingly.
3. Results and Discussion
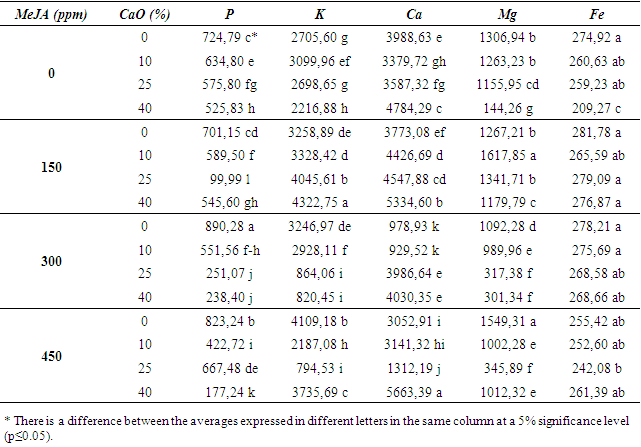
- The performances of the effects of MeJA application at different lime concentrations on three different American grapevine rootstocks are presented in the tables below on the basis of rootstock genotypes. The lowest weighted shoots of 5 BB in assessing shoot weights appear to be from plants grown in the highest lime-containing pots without the application of MeJA (Table 1). Here, the impact shown by lime without the MeJA stands out. No statistical difference in terms of shoot lengths of these plants was identified. Similarly, no statistical differences were detected in terms of the ANLPS. However, although any difference was not detected, there are numerical fluctuations between the treatment groups and it is thought that the differences will also be seen statistically if the administered scales of lime and MeJA doses are wider.
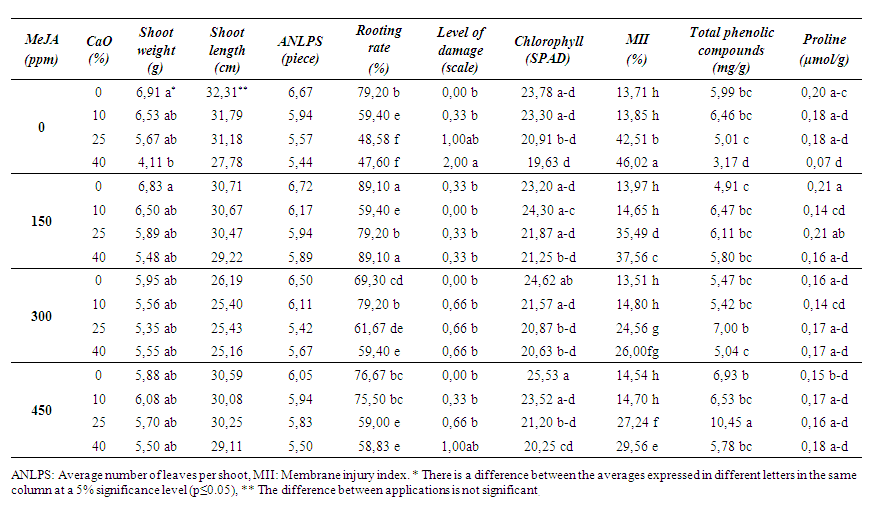 | Table 1. Effects of MeJA on some physical and biochemical properties in 5 BB rootstock |
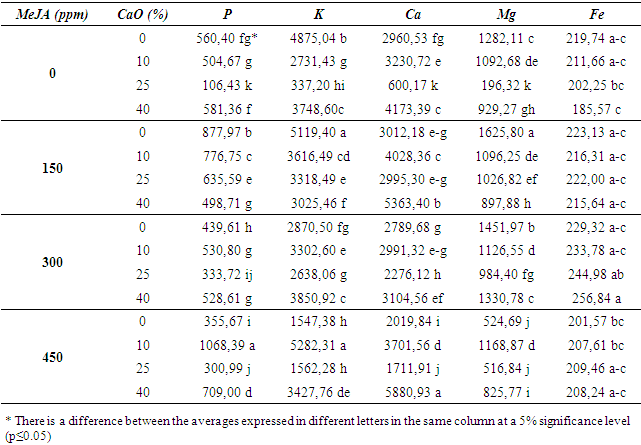
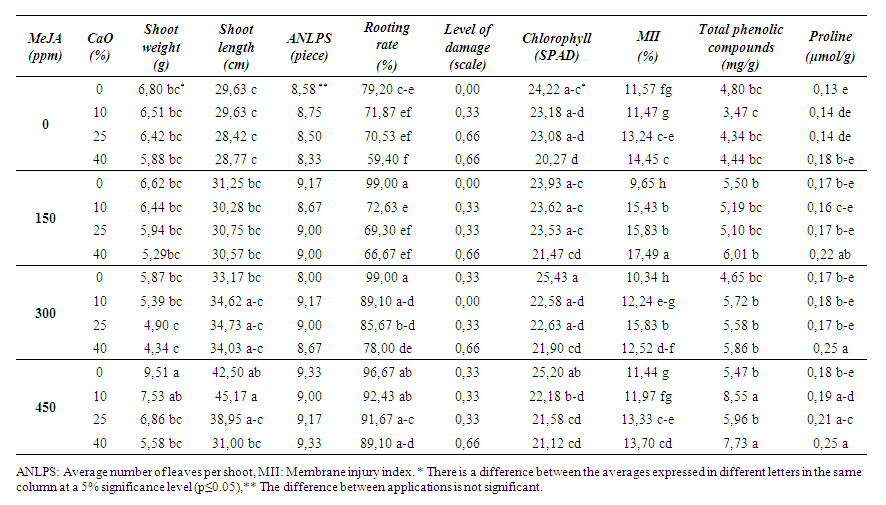 | Table 2. Effects of MeJA on some physical and biochemical properties in 41 B rootstock |
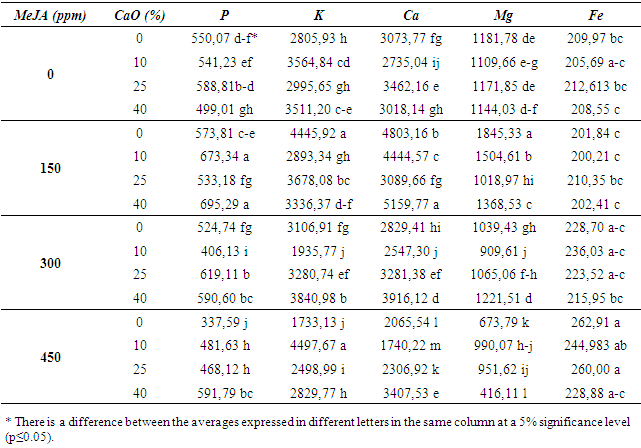
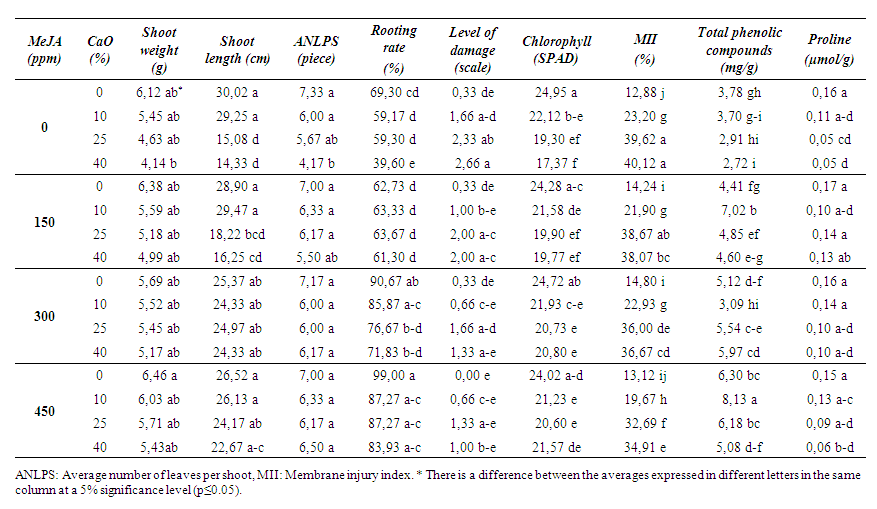 | Table 3. Effects of MeJA on some physical and biochemical properties in 1103 P rootstock |
|
|
|
4. Conclusions
- The high lime content is a condition seen in most of the land used in agriculture. Although vine is thought of as a plant that can more easily adapt to limy soils compared to many cultural plants, the negatives from lime are reflected in yields and quality. The most commonly used method for reducing the lime content of soils is the application of micronized sulfur, which is impractical if it is to be repeated and show its effect later, especially when it needs to be applied to vast lands. Therefore, approaches to increase the resistance of the plant to the stress environment are important. It is known that the application of jasmonate, a natural plant hormone, as a regulator of growth increases plant tolerance in a wide range of stress environments. Here, the effects of MeJA, a jasmonate, on the physical and biochemical properties of vine rootstocks, which have different resistance to lime, as well as their capacity for mineral uptake, were investigated, and the resulting data indicated the potential for use of MeJA in countering this stress. It is also thought that it would be effective to try different doses of MeJA, especially in different culture plants whose sensitivity to lime is known.
ACKNOWLEDGEMENTS
- This work was financially supported by The Scientific and Technological Research Council of Turkey-2209-A- Research Project Support Programme for Undergraduate Student.
Conflict of Interest
- The authors declare that they have no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML