-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2021; 11(1): 14-19
doi:10.5923/j.plant.20211101.03
Received: Sep. 21, 2021; Accepted: Nov. 1, 2021; Published: Nov. 28, 2021

Analyzes of Phytochemicals, Antimicrobial Activities and Minerals in the Leaves of Adansonia suarezensis Diego Suarez
Ramahaimananjato Malaza1, Zafilaza Armand2, Landy Soambola Amelie3
1University of Madagascar Antsiranana, Faculty of Sciences, Mention Science of Nature and Environment, Antsiranana, Madagascar
2University of Madagascar Antsiranana, Faculty of Sciences, Mention Science of Nature and Environment, Option Biochemistry, Antsiranana, Madagascar
3University of Madagascar Antsiranana, Faculty of Sciences, Mention Nature and Environmental Science, Marine Science Option, Antsiranana, Madagascar
Correspondence to: Ramahaimananjato Malaza, University of Madagascar Antsiranana, Faculty of Sciences, Mention Science of Nature and Environment, Antsiranana, Madagascar.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Adansonia suarezensis is an endemic plant of Diego Suarez. This plant has medicinal and cosmetic properties. In our research, we analyzed the phytochemicals, microbial activities and minerals in the leaves. The ash content of the leaves used is 21.32g / 100g. Phytochemical analysis shows the significant amount of alkaloids, tannins and polyphenols in the leaves. Mineral analysis proves that Adansonia suarezensis leaf is rich in Calcium (5.29g / 100g) and potassium (2.08g / 100g). The antimicrobial test shows that Adansonia suarezensis has significant activity in the development of bacteria such as Pseudomonas, Echerchia coli, and Streptococus pneumoniae with inhibiting halos 15, 14 and 14 mm in diameter. But it reacts with other bacteria such as Salmonella enterdis with halo 12mm and Candida albicans 11mm. So the leaves of Adansonia suarezensis are very effective natural bactericides in the medicinal field.
Keywords: Adansonia suarezensis, Leaf, Analysis, Phytochemical, Antimicrobial
Cite this paper: Ramahaimananjato Malaza, Zafilaza Armand, Landy Soambola Amelie, Analyzes of Phytochemicals, Antimicrobial Activities and Minerals in the Leaves of Adansonia suarezensis Diego Suarez, International Journal of Plant Research, Vol. 11 No. 1, 2021, pp. 14-19. doi: 10.5923/j.plant.20211101.03.
Article Outline
1. Introduction
- The research would take place in the northern part of Madagascar and more precisely in the District Diego II region DI.A.NA. In this region there are many tourist sites and different national parks. The DI.A.NA region occupies 50% of currency entry in Madagascar in the export of commercial products, such as Vanilla, Cocoa, precious stone and fishery product. There are many hotels to be found in the area's long coastal beach. But in front of this development in the region, it causes a big problem in biodiversity. It destroys the marine ecosystem, flora and fauna in the natural environment. In the region of DI.A.N.A, baobabs are very remarkable trees from the point of view of morphology and use. They are trees with multiple and sacred uses. From an adaptation point of view, they are trees adapted to drought. They are found in dry and semi-arid areas as well as in the far north of Madagascar, thanks to their water storage capacities. According to forecasts by climatologists, Madagascar should be subject to significant climate change. But the different species of baobabs are not equal in the face of the changes in temperature and precipitation announced. Concerning the baobab Adansonia suarezensis, it is an endemic species of the forest of the Montagne des Français, District Diego II Region DIANA [1]. According to the Red List of the International Union for the Conservation of Nature (IUCN), this species is among the three baobabs considered in danger of extinction, yet this species has technological, mechanical, medicinal and ecological characteristics. important. So in the face of this scourge the goals of the various researches are oriented towards protection, knowing the active principles, chemical molecules and mineral elements in the leaves of Adansonia suarezensis.a) Problem:Adansonia suarezensis is very fragile; 90% of the plant is found only in the District of Diego II. The problem with this species is the lack of natural regeneration in its current natural range. One such threat is the lack of natural regeneration or the low rate of regeneration. So the number of Baobab Adansonia suarezensis is very limited in Madagascar due to developmental conditions like temperature, soil, humidity and rainfallb) Research objective:The research objective is to:1. Know the different chemical molecules and nutrients contained in the leaves of the species studied.2. Know the effect of using this plant medicinally.
2. Study Zone
- The French mountain known as Ambohit’Antsingy is located in District Diego II by the Nosy lonjo sea. It is characterized by a fossiliferous Eocene limestone massif. It has a relief oriented North-West / South-East whose peaks are located between 350 m and 425 m of altitude and the brutality of the rocky escarpments gives it a real aspect of "Mountain". It is a very recognized tourist site in terms of fauna and flora [1].The forest of the Montagne des Français belongs to the type of dense dry forests defined in 1956 by the Yangambi Congress. It is divided into three types of forest:- Dense semi-deciduous dry forest.- Secondary education.- Dense subhumid forest.
3. Adansonia suarezensis
3.1. Distribution Area of Adansonia suarezensis
- Adansonia suarezensis grows in a very restricted area in the extreme north of Madagascar. The distribution of this species is between Cape Amber and the town of Diego Suarez, Montagne des Français in the East, Littoral West to Cape Saint Sebastian [2].
3.2. Description of Adansonia suarezensis [4]
3.2.1. Trunk
- Adansonia suarezensis is a baobab tree from Madagascar. It is very big; it is at least 25 meters high and 2 meters wide. The tree can live for thousands of years. Its trunk is smooth, cylindrical and shrinks from bottom to top. The bark is smooth, varying in color from burgundy red at the seaside to gray in the land. The roots of Adansonia develop in a bulb; first in depth, and then in width. Baobab is also the only tree capable of regenerating its bark directly from the surface of exposed wood. The tree typically grows in dry, rocky, well-drained soils. Its soft, spongy wood soaks up water during the rainy season, so as to retain a considerable amount of water that will be required during the dry season.
3.2.2. Sheet
- The palmate leaves up to 16 cm long stalked and 7-9 oblong leaflets 10.5-15.5 cm long by 3-6 cm wide, green in color, broad and glabrous with entire deciduous margin (falls during the dry season). In the fall the leaves will start to fall, and by the end of December your Baobab will be completely dormant. When the tree has lost all its leaves, the branches look like roots; In March, when the temperature rises, the leaves will reappear.
3.2.3. Flower
- Characterized by solitary flowers, white or red, small, erect with greenish-brown sepals, rolled up 7 cm long by 2 cm wide and 5 narrowly obovated petals 7-8 cm long by 2 cm wide, stamens numerous white 7-8 cm long, welded in a tube, at the base 0.7-1.2 cm, white style.
3.2.4. Fruit and Seed
- Indehiscent dry fruits oblong, irregular, long and typically costulate, 20-25 cm long by 10 cm in diameter with a hard pericarp. The seeds of Adansonia suarezensis are the largest of all baobab species.
3.3. Use of Adansonia suarezensis
- Adansonia suarezensis a multipurpose tree. It is used like all other medicinal plants. The bark is used to treat Diabetes, used as herbal tea, bath water for babies who are underweight or abnormal (Fakabe).
3.4. Classification of Adansonia suarezensis [4]
- Suarez's Baobab (Adansonia suarensis) is a tree species of the Bombacaceae family according to the classical classification, or of the Malvaceae according to the phylogenetic classification.
3.4.1. Classical Classification
- Reign: PlantaeUnder Reign: TracheobiontaDivision: MagnoliophytaClass: MagnoliopsidaSub Class: DilleniidaeOrder: MalvalesFamily: BombacaceaeGenus: AdansoniaSpecies: Adansonia suarezensis
3.4.2. Phylogenetic Classification
- Phylum: AngiospermClass: DicotyledonousSub Class: RosidsOrder: MalvalesFamily: MalvaceaeGenus: AdansoniaSpecies: Adansonia suarezensisVernacular names: Bozy Boringy, Fontana, Ringy, Sefo
4. Materials and Methods
4.1. Mineral Analyzes
- In this experiment, the plant materials used are leaves collected from a young individual of Adansonia suarezensis developed in the forest of the Montagne des Français. The sample used is the vegetable powder obtained after grinding the leaves.The powder is placed in a muffle furnace at 550°C to obtain a white ash which contains the minerals.The contents of minerals Ca, Mg, K, Na are determined by atomic absorption spectrophotometry. After humidification, 5 to 25 ml of concentrated hydrochloric acid are added. The suspension is then brought to a boil, then filtered. The phosphorus level is determined by colorimetry or by spectrophotometry at 560 nm [11].
4.2. Phytochemical Screening
- The plant material is extracted from the leaves and liana. The plant materials were harvested in March in the dense forest of Ampondrabe. These organs were washed in running water and oven dried at 50°C for 5 days. Then the plant materials were placed in the rotavapor for the distillations and extraction of essential oils. The tests for the detection of large groups of chemical compounds focused on the residues. We made use of the analytical techniques described in the practice papers [11].
4.2.1. Detection of Sterols and Polyterpenes
- Sterols and polyterpenes were detected in residues (R1) by the liebermann reaction. An aliquot of the residue is dissolved hot in 1 ml of acetic anhydride in a capsule, then taken up in a test tube into which 0.5 ml of concentrated H2SO4 is poured. The appearance of a purple color that turns blue and then green indicates a positive reaction [11].
4.2.2. Tannin Detection
- Tannoids (hydrolyzable tannins) and true tannins (non-hydrolyzable or condensed tannins) are polymers of polyphenols. The latter were demonstrated by the reaction with iron (III) chloride in the crude extracts (S1-S5). A few drops of a 2% aqueous FeCl3 solution are added to 2 ml of crude extract. The appearance of a blue-black or green-black coloration respectively indicates the presence of tannoids or true tannins. True tannins were demonstrated in the residues (R3-R5) by Stiasny's reagent. 15 ml of Stiasny's reagent (30% CH2O in concentrated 2/1 HCl) are added to an aliquot of residue taken up in MeOH and then evaporated. Then, the formation of precipitate in the form of flakes after cooling, indicates a positive reaction.The true tannins were also demonstrated when hot in the presence of concentrated HCl in an aliquot of residue taken up in 2 ml of distilled water, a few drops of concentrated CHl are added, the whole is heated in a boiling water bath. Formation of a red precipitate indicates a positive test. The tannoids were detected in the residues (R3-R5): to the filtrates of the reaction masses saturated with CH3COONa are added a few drops of a 2% aqueous FeCl3 solution. The reaction is positive if a blue-black tint appears [11].
4.2.3. Detection of Flavonoids
- Flavonoids were evidenced in the residues (R1-R5) by the reaction with cyanidin. To an aliquot of residue dissolved in 5 ml of hydrochloric ethanol (2: 1, v / v) are added two to three Mg shavings (or 30-50 mg of Zn powder) and a few drops of isopetanol. The appearance of an intense pink-orange or purplish coloration (red or red-orange with Zn) indicates a positive reaction [14].
4.2.4. Quinone Detection
- Quinones were demonstrated in the residues (R1 - R5) by Borntraëger's reagent. An aliquot of the residue dissolved in 5 ml of HCl diluted to 1/5, is heated in a boiling water bath for 30 min, then extracted with 20 ml of CHCl3 after cooling. To the organic phase are added 0.5 ml of NH4OH diluted to 50%. The appearance of a tint ranging from red to purple indicates a positive reaction.
4.2.5. Saponin Detection
- The saponins were demonstrated in the residues (R1 - R5) by the foam test, then their presence is confirmed by the blood test and by the determination of the optical density (OD). The residues are taken up in 5 ml of distilled water, then introduced into a test tube. The test tube is shaken vigorously. The formation of a stable foam (height greater than 1 cm), persisting for 1 hour, indicates the abundant presence of saponins. The blood test was performed on the aqueous extracts of R4 and R5. Then in a test tube containing 2 ml of fresh animal blood dissolved in physiological solution (0.9% aqueous NaCl solution), a few drops of aqueous extract are added. Observation of discoloration from a control tube indicates a positive test. Then in 3 test tubes is introduced 1 ml of blood solution (1 ml of blood in 25 ml of isotonic solution). One of the tubes serves as a control. In each of the two remaining ones, are added respectively 5 and 10 drops of extracts to be tested. After homogenization, the content of the test tubes is centrifuged for 10 min at 2000 g, then the OD of each supernatant collected is measured using a colorimeter (wavelength 420 nm) [14].
4.2.6. Detection of Reducing Sugars
- The reducing sugars were demonstrated in the crude extracts (S1 - S5) by Fehling's reagent, then their presence was confirmed by the Tollens test. To perform the Fehling test, 5 ml of crude extract are added 5 ml of Fehling's liquor. The formation of a brick red precipitate after 2-3 min of heating in a 70°C water bath indicates a positive reaction. The detection of reducing sugars by the Tollens test consisted in adding to 5 ml of crude extract, 5 ml of Tollens reagent. The formation of a silver mirror after a few minutes indicates a positive reaction [15,16].
4.2.7. Coumarin Detection
- Coumarins were detected in the residues (R1 - R5) by the reaction on the lactonic ring. 2 ml of ethanolic solution obtained from each residue are introduced into 2 test tubes. In one of the test tubes, 0.5 ml of 10% NaOH are added, then the test tubes are heated in a water bath until boiling. After cooling, are added to each test tube 4 ml of distilled water. If the liquid in the test tube to which the alkaline solution has been added is transparent or more transparent to the liquid in the control test tube (without an alkaline solution), then the reaction is positive. By acidifying the transparent solution with a few drops of concentrated HCl, it loses its yellow color, becomes cloudy or a precipitate forms [15,16].
4.2.8. Protein Detection
- The proteins were evidenced in the residues (R1 - R5) by the reaction of biuret. To an aliquot of the residue dissolved in 2 ml of 20% aqueous NaOH in a test tube are added 2-3 drops of 2% aqueous CuSO4 solution. The appearance of a purple color, sometimes with a reddish tinge, indicates a positive reaction [15,16].
4.2.9. Alkaloid Detection
- The alkaloids were demonstrated in the residues (R1 - R5) with the Dragendorff and Burchard reagents (precipitation reagents): 0.1 g of residue is taken up in 6 ml of 60% ethanol, then distributed in 2 test tubes. In the first tube, are added 2 drops of Dragendorff reagent. The appearance of an orange-red or reddish-brown precipitate indicates a positive test. In the second tube, are added 2 drops of Burchard's reagent. The appearance of a brown precipitate indicates a positive test [15,16].
4.3. Antimicrobial Activity
- In the test for antimicrobial activity with Adansonia suarezensis many of the germs are used. These are Gram - and Gram + germs as well as pathogenic unicellular fungi. They came from the Environmental Microbiology Laboratory (LME) of the National Center for Environmental Research (CNRE).To the extent that antimicrobial activity is determined, agar diffusion is used. A Petri dish containing an agar medium (culture inoculated into 5 ml of molten agar to cover the agar nutrient medium) is seeded over its entire surface by strain of bacteria and fungi studied. The extract of Adansonia suarezensis is deposited on filter paper discs which are then placed on the surface of the agar. During incubation the extract diffuses from the filter paper into the agar (24 to 48 h incubation); The further away from the disc, the greater the concentration of the extract deprived. At a certain distance from the disc, the Minimum Inhibitory Concentration (MIC) is reached. Beyond this limit bacteria and fungi develop while, closer to the disc, growth is inhibited. A zone of inhibition is created with a diameter proportional to the amount of antimicrobial agent deposited on the paper disc, the solubility of that agent, the diffusion coefficient and the overall activity of the agent.Here is the list of germs used during the series of tests with their characteristics [17].
4.3.1. Gram - Bacteria
- - Escherichia coli: Called "colibacillus", this bacterium is the normal host in the intestines of humans and warm-blooded animals. It is a fecal coliform, an indicator of fecal contamination in water and food. It is sought after in butcher's meats, fishery products, raw and fermented milks.The strains responsible for infections in humans are different from those found in the intestinal flora. They are particularly involved in diarrheal syndromes in adults and young children in developing and developed countries.They are classified as group 2 biological pathogens, meaning that they can cause disease in humans and pose a danger to workers, but their spread in communities is unlikely. In addition, there is effective prophylaxis and treatment [20].- Salmonella enteridis: it is an intestinal parasitic bacterium of the vertebrae. Its pathogenic strain is responsible for gastroenteritis during individual or collective food poisoning. These conditions called salmonellosis are manifested by diarrhea, vomiting and fever that appear 8 to 10 hours after eating contaminated food. This bacterium is also classified as a pathogen 2 [20].
4.3.2. Gram + Bacteria
- - Bacillus cereus: Living in soil and water, this bacterium can survive in the environment as spores. It can contaminate plant foods and many cooked meals. It is one of the microbes that are necessarily sought in raw vegetable products, peeled and cut, those ready to use, sprouted seeds, raw vegetable and fruit juices [20].- Staphylococcus: Staphylococci are spherical cocci isolated in clusters or diplococci. They are immobile, facultative anaerobes in general and they grow in nutrient agar or soybean trypcase agar at 37°C and pH ranging from 7.2 to 7.4. They are of human or animal origin (poultry, cattle, etc.) and are pathogenic in group 2. The pathogenicity is:- by virulence: Staphylococci produce surface proteins and enzymes including free coagulase and thermonuclease, thus causing a wide variety of infections including boils, pneumonia,….- by toxinogenesis: staphylococci produce various toxins including enterotoxins causing individual or collective poisoning due to contaminated food. This food poisoning manifests itself within 2 to 4 hours as nausea, abdominal pain, repeated vomiting and diarrhea lasting 24 to 48 hours [20].- Enterobacter cloacae: enterococci are oval cocci, isolated or in diplococci, in chains or immobile chains and optional anaerobes. They are grown at 37°C and at pH 7.2 on standard media.These are ubiquitous germs found in soils, freshwater, marine and living plants as commensals in the intestines of humans and animals. They can be pathogenic for humans and animals by group 2. They are responsible for various and formidable human opportunistic infections such as endocarditis, septicemia, meningitis and urinary tract infections [20].
4.3.3. Pathogenic Yeast Fungi
- Candida albicans: The colonies of this yeast are creamy white to beige convex on Sabouraud agar. It produces blastospores and chamydospores. It is sought after during microbiological control of cosmetic products. Pathogenic to humans (group 2) and animals or an agent of mycosis of the skin or mucous membranes [27,28].
5. Results and Interpretations
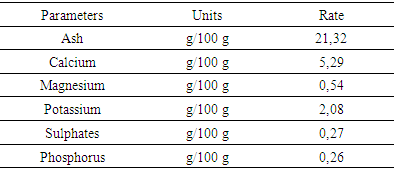
5.1. Mineral Analysis Results
|
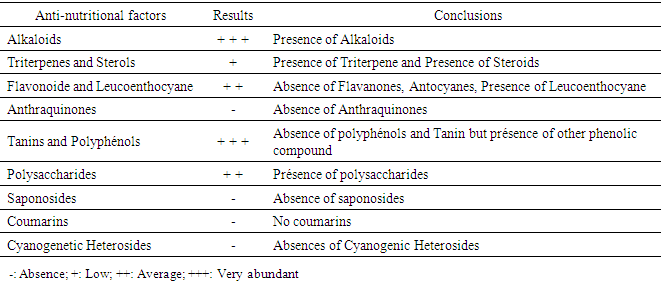
5.2. Phytochemical Results
|
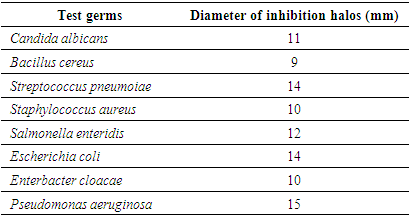
5.3. Antimicrobial Activity Results
- Adansonia suarezensis has an important activity in the development of bacteria such as Pseudomonas aeruginosa, Escherichia coli and Streptococcus pneumoiae with inhibition halos 15, 14 and 14 mm in diameter. The different types of molecules in the leaves of Adansonia suarezensis are able to kill bacteria. But it reacts with other bacteria such as Salmonella enteridis with halo 12mm and Candida albicans 11mm.
|
6. Discussion
- The presence of minerals like Calcium, Potassium with high levels 5.29 g / 100g and 2.08g marks the importance of Adansonia suarezensis at the level of medicinal plant, cosmetic. Roughly speaking, the leaves of Adansonia suarezensis rich in essential minerals like, magnesium, potassium, calcium, phosphorus and sulphates.Phytochemical analyzes show the significant presence of alkaloids and other phenolic compounds. Adansonia suarezensis leaves also contain Polysaccharides, Leucoenthocyanin, Triterpene and Steroids in medium amounts. Based on the lives of people around this site.The microbiological test also shows the antimicrobial activity of Adansonia suarezensis leaves on bacteria and fungi. It is able to inhibit the growth or kill bacteria like Pseudomonas aeruginosa. It is a bacteria that is very resistant to different types of antibiotics or to the medicinal plant. But according to our tests, 15mm the diameter of the inhibition halo caused by the leaf of Adansonia suarezensis; therefore, the result gives the agility of Adansonia suarezensis in the bactericide. Adansonia suarezensis also reacts in other bacteria such as Escherichia coli, Streptococcus pneumoiae and Salmonella enteridis with 14,14- and 12-mm halo inhibition. So, the leaves of Adansonia suarezensis are very effective natural bactericide in the medical field. This is why the population around the site uses the baobab leaves of Adansonia suarezensis as the most widely used drug. The leaves are mixed with the peel to complete the effectiveness. However, Adansonia suarezensis very endemic in the French mountains so it is very protected.
7. Conclusions
- Adansonia suarezensis is a very endemic baobab of Madagascar and mostly it is only found in the French mountains. It plays an important role in the life of the populations surrounding the site due to the presence of different minerals such as calcium, magnesium, potassium, phosphorus and sulphites. It is used as a first-class remedy to cure the skin, face and different types of infectious diseases. Traditional therapists use Adansonia suarezensis to heal the sick.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML