-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 10(3): 53-60
doi:10.5923/j.plant.20201003.02

Effect of Arbuscular Mycorrhizal Fungi Used as Biofertilizer for the Vegetative Propagation of Prunus africana (Hook.f.) Kalkman
Kamko J. D.1, Tchiechoua Y. H.1, 2, Ngonkeu E. L. M.1, 3, Nzweundji J. G.4, Tchatat M.3, Eloumou D.5, Mam C. E.1, Chamedjeu R. R.1, 2, Tekeu H.1, Lessa F. T.1, Foko B.1, Damdjo A.1, Ngo Ngom A.1, Boyomo B.1
1Department of Plant Biology, Faculty of Science, University of Yaoundé 1, Yaoundé, Cameroon
2Department of Molecular Biology and Biotechnology, Pan African University, Institute of Basic Sciences, Technology and Innovation, Nairobi, Kenya
3Institute of Agricultural Research for Development, Nkolbisson, Yaoundé, Cameroon
4Institute for Medical Research of Medicinal Plants Studies, Yaoundé, Cameroon
5Congo Basin Institute, Awae, Yaoundé, Cameroon
Correspondence to: Kamko J. D., Department of Plant Biology, Faculty of Science, University of Yaoundé 1, Yaoundé, Cameroon.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Prunus africana is a medicinal plant species whose exploitation became abusive over the years due to the high properties of its bark. It faces multiple challenges: the recalcitrance of its seeds and the irregular fruiting which makes its sexual regeneration difficult. Therefore, asexual regeneration has been set as the appropriate corridor for its domestication. Nonetheless, this way has encountered difficulties, particularly in the continuous growth of cuttings due to the weak development of its root systems. The optimization of plant mycorrhizal symbiosis is a key factor to overcome this constraint. This justifies the aim of this work which was to develop a method of producing P. africana cuttings inoculated with its native Arbuscular mycorrhizal fungi (AMF) to improve its regeneration. To undergo this purpose, soils samples were collected in three Mounts (Cameroon, Oku and Manengouba), then, isolation and characterization of AMF species was done after the fungi trapping culture followed by a pre-germination, multiplication and evaluation of their growth efficiency. To see their real efficiency on Prunus root system, each AMF species multiplied has been used as biofertilizer on Prunus cuttings in a frame, where indol butyric acid (AIB) was used as positive control and water was applied as a negative control. The result revealed the presence of six species of AMF belonging to the genera Gigaspora (1), Glomus (3) and Acaulospora (2). Each AMF species showed a great capacity of growth improvement and self-multiplication. Inoculated AM fungi increased the root density of Prunus africana cuttings with an average root number of 35 for the more efficient species (Gigaspora margarita), compared to the positive control which had an average root number of 10 and the negative control 4. These results can contribute to the improvement of P. africana regeneration.
Keywords: Domestication, Cuttings, Biofertilizer, Symbiosis, Root density
Cite this paper: Kamko J. D., Tchiechoua Y. H., Ngonkeu E. L. M., Nzweundji J. G., Tchatat M., Eloumou D., Mam C. E., Chamedjeu R. R., Tekeu H., Lessa F. T., Foko B., Damdjo A., Ngo Ngom A., Boyomo B., Effect of Arbuscular Mycorrhizal Fungi Used as Biofertilizer for the Vegetative Propagation of Prunus africana (Hook.f.) Kalkman, International Journal of Plant Research, Vol. 10 No. 3, 2020, pp. 53-60. doi: 10.5923/j.plant.20201003.02.
Article Outline
1. Introduction
- Prunus africana (Hook.f.) Kalkman commonly known as Pygeum, is a mountainous tree species from tropical Africa (Betti et al., 2014). It grows on volcanic soil and in cool climates at high altitude between 900 and 3000 m (anonymous, 2015). This plant is much better known for the healing properties of its back extracts, used for the manufacture of more than nineteen drugs, sold by European and American companies for more than 30 years for the treatment of benign prostatic hyperplasia (Cunningham et al., 2002), as well as many other related disorders such as cancer. However, the plant produces a low quantity of secondary metabolites in their natural environment and this has justified the large exploitation of its bark to obtain the active compounds essential for global therapeutic needs. Thus, a world demand of 4000 tons per year (Cunningham et al., 2002; Jimu, 2011) has resulted to an increase in the species’ mortality rate due to barking (Sunderland and Tako, 1999). Domestication, the creation of large-scale nurseries and plantations are among other techniques put in place in order to maintain the availability of P. africana bark (Cunningham and Mbenkum, 1993). Nonetheless, this domestication remains limited to the problem of seed availability and adaptation of cuttings to weaning during transplantation, due mainly to the weak development of their root system. This is when rhizospheric microorganisms such as arbuscular mycorrhizal fungi (AMF) come into play, whose plant growth promotion abilities help to boost the development of the plant root system (Barea et al., 2002). They play a key role in the production of phytohormones such as AIA (natural auxin) and ZR (Zeatin Riboside) (Torelli et al., 2001; Ludwig-Müller and Güther, 2007) and ensure root system development (Morrison et al., 1993; Johansson et al., 2004; Smith and Read, 2010).Arbuscular Mycorrhizal fungi (AMF) are symbiotic associations between plant and fungi. AM fungi get almost all their carbon as glucose from the host plant (Bago et al., 2000). AMFs are obligate biotrophs, they need a plant partner to support their nutrition and complete their development cycle. However, the only phase in their life cycle where the fungus does not depend on the host plant is that of spore germination (Bago et al., 2000), as they use their accumulated carbon reserves during their development and can therefore be induced in vitro under appropriate conditions (appropriate substrate) before their transfer to a young root system of a host plant. In return, AMF bring tremendous benefits to plants. They provide better nutrition in minerals such as phosphorus (Harrison, 2005; Helgason and Fitter, 2005; Feddermann et al., 2010), nitrogen and other essential cations such as zinc, copper, manganese and iron (Liu et al., 2000).The presence of Arbuscular Mycorrhizal fungi (AMF) in the rhizosphere of Prunus plants amplify their root system. By obtaining all their carbon substances from their host plant, AM fungi in return with the help of their hyphal network, draw minerals and water from the soil, thus increasing their hosts’ nutritional resources, mainly for phosphorus which also protects them against saline stress and several types of pests (Bago et al., 2000; Helgason and Fitter, 2005). The work of Njeudeng (2001) and Nzweundji (2015) on the mycorrhizal status of P. africana in Cameroon, revealed that the plant is mycotrophic. It would therefore be wise to scrupulously define the mycorrhizal fungi species active in the rhizosphere of the plant in order to make their production profitable to the domestication of this plant. Hence the general objective of this study was to develop a method of production of P. africana cuttings inoculated with native mycorrhizal fungi in order to improve its regeneration and domestication. Specifically, it was to morphologically describe the mycorrhizal fungi subservient to P. africana then multiply them for production of plant-specific fertilizers as well as the evaluation of their efficacy on plant growth promotion.
2. Materials and Methods
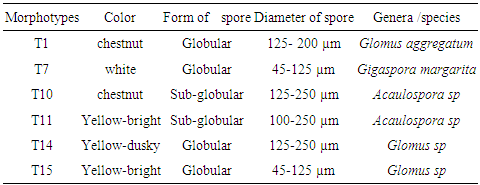
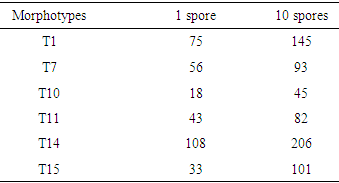
- Description of the sampling sites Samples (soils and fine roots of Prunus) were collected from three sites: Mount Cameroon, Mount Manengouba and Mount Oku. Mount Cameroon is located in a wet forest area of the agro-ecological zone IV at 4100 m above sea level, with monomodal rainfall and a black-colored soil, furnished with granitic crystals; the geographical coordinates of sampling site: 4°44 'N, 9°32' E. Mount Manengouba is located at an altitude of 3211 m, humid forest area of the agro-ecological zone IV, also with single-mode rainfall, with a very dark black soil; the geographical coordinates of sampling site: 5° 6 'N, 10° 7'E. Mount Oku is located at 2000 m altitude; in the western highland area of the agro-ecological zone III, with a slightly black (brown) soil; the geographical coordinates of sampling site: 7° 15 'N, 11° 15' E.The method used for soil sampling was that of Sieverding (1991). For each site, an area of one hectare was delimited and subdivided into 20 quadrants measuring 25 × 20 m2. In each of these quadrants, one plant of P. africana was randomly selected, the roots were collected between 0 to 45 cm together with a soil sample around the rhizosphere of the tree using an auger. At each quadrant, a total of 500 g of soil sample were collected and soil samples from the same site were mixed to make a composite sample of 10 kg of soil per site. The roots were sampled at the same points of soil sampling at a rate of 8g per tree. The collected roots from the same site were also mixed and stored in 50% of alcohol solution.Isolation and characterization of AMF sporesTo assess the association between Prunus and AM fungi present in their rhizosphere, root staining was done by a modified method described by Ngonkeu (2009). The roots were cut and introduced into test tubes, 10% KOH was added and the mixture was allowed to stand for 15min at 90°C in a water bath. Then 30% of hydrogen peroxide (H2O2) was introduced into the mixture for root thinning. The roots were removed and rinsed three times with distilled sterile water and then soaked in 10% HCl solution for 15 min at 90°C in a water bath to remove excess of KOH so as to prepare the roots for staining. The roots staining was done with 0.01% methyl blue heated at 90°C in a water bath for 35 min and rinsed three times with distilled sterile water before observation using an optical microscope (WILD M2B, Germany).To optimize the density of the AM fungi spores, present in the soil samples collected, fungi trapping culture was done following the method described by Ngonkeu (2009). A 3L jar was subsequently filled at 1/3 with sterilized sand, completed to 2/3 volume with 1kg inoculum (collected soil) and then filled with sterilized sand. The sterilization was done at 121°C for 1h in an autoclave (model PTS-B100L). Soil samples from each site were used to fill 10 pots on which two highly mycotrophic plants: Vignia onguiculata and Sorghum bicolor were planted and disposed in a greenhouse, where they were allowed to grow for three months, during which the plants were regularly watered. After three months of development, these plants were subjected to water stress in order to stimulate the sporulation of mycorrhizal fungi in association with them.To isolate the AMF spores obtained after trapping, extraction was performed according to the method proposed by Brundrett and al., (1996). After homogenization of the substrate, 100 g were removed and introduced into a beaker of 1000 ml. 300ml of distilled water was added followed by stirring of the mixture then allowed to stand for 15 seconds. Four sieves were arranged in decreasing order of mesh size 710 μm, 250 μm, 125 μm and 45 μm in which the supernatant of each mixture was filtered. Then the content of the last three sieves was collected, washed and observed with stereo microscope (WILD M2B, Germany). The observed spores were characterized and identified according to the Morton (1988) method based on size, shape, hyphae, presence or absence of the suspensory bulb, spore-forming saccules, germination loops, and colors. Then they were grouped by species and named Tx (x being a number assigned in the order of discovery) then counted according to the method described by Ngonkeu (2003).In order to optimize the symbiosis between the identified AM fungi and the tall plant (Soghum bicolor), a purification and pre-germination of the spores on agar media were conducted following the method described by Ngonkeu (2009). In brief, the identified spores were introduced into Eppendorf tubes for sterilization with 3ml of 2% Chloramine T followed by vortexing for 20min. After removal of chloramine T, the spores were rinsed three times with sterile distilled water. 3ml of Streptomycin 0.025% was then introduced into the tubes; the mixture was stirred for 20 minutes followed by 3 times rinsing with sterile distilled water for 15 min. The disinfected spores were sown base on morphotype in Petri dishes containing agar medium (0.7%) at the rate of 4 spores per dish with three replicates. The Petri dishes were then sealed and incubated in the dark for at least 4 days at 30°C.Evaluation of the identified AMF efficiency on the vegetative development of Prunus africanaTo optimize and intensify the activity of the identified AM fungi, purification of the strains was performed using the method described by Ngonkeu (2009). The pre-germinated spores were inoculated on the roots of a mycotrophic plant (Sorghum bicolor) and then gently introduced into 3L pots filled with sterilized sand. In order to test if the AM fungi efficacy of promoting growth dependent on the number of spores initially present in the medium, an experiment was conducted with six treatments and one negative control without inoculation. One treatment consisted of 6 Sorghum plants (Bafia’s variety) inoculated with one AMF specie with two variables (1 spore and 10 spores) and three replicates. The plants of a treatment (same AMF species) were grouped in a tray of 8m2. The negative treatment consisted of plants not inoculated with AM fungi. The plants were regularly watered by capillary action for three months and growth parameters such as stem length, number and leaf surface area were recorded to verify the effectiveness of the mycorrhizal symbiosis. Then the plants were subjected to water stress to stimulate sporulation. Finally, extractions were carried out to confirm the multiplication of spores and the reliability of obtaining a biofertilizer whose efficiency could be evaluated on Prunus cuttings.The efficacy of the identified AM fungi strains was evaluated on the vegetative propagation of Prunus cuttings in a greenhouse following the method described by Leakay et al., (1994). A treatment consisted of Prunus cuttings sown on substrates (sand) inoculated with a biofertilizer (spores of AM fungi). Indol Butyric Acid (AIB) which is a root growth inducing hormone, was used as a positive control at a dose of 75 μg per cutting. The negative control included cuttings grown on a substrate with no AM fungi applied (no biofertilizer). The experimental unit consisted of 15 cuttings. After 25 days of propagation, the cuttings of the different treatments were evaluated on the basis of their root density in comparison with that of the controls and the experiment was repeated three times.
3. Results
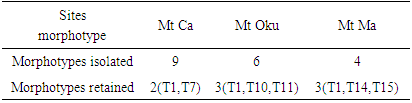
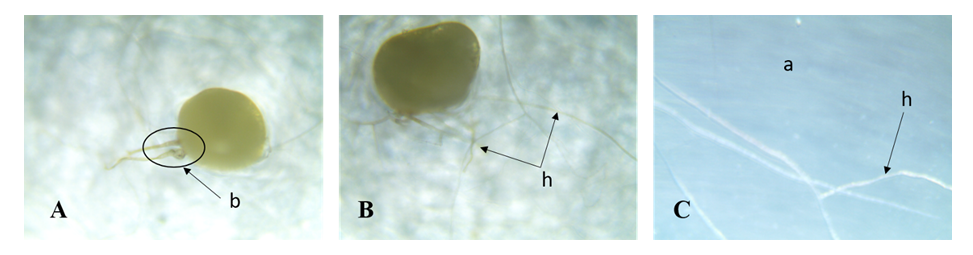
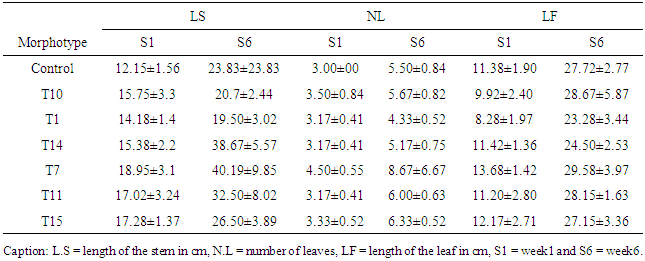
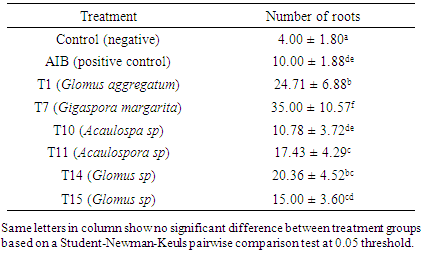
- Isolation and characterization of Arbuscular Mycorrhizal fungi sporesThe soil samples collected showed different characteristics depending on their sampling site. Thus, we obtained more morphotypes in Mount Cameroon site (9) compared to Mount Oku (6) and Mount Manengouba (4). Root staining revealed the presence of mycorrhizal fungus propagules such as vesicles and hyphae inside the roots, characteristic of endomycorrhizal symbiosis (fig. 1).
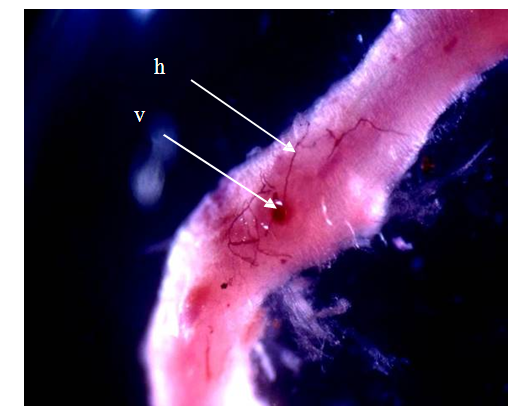 | Figure 1. Arbuscular Mycorrhizal fungi structures (h: Hyphea and v: Vesicles) in roots fragments of P. africana |
|
|
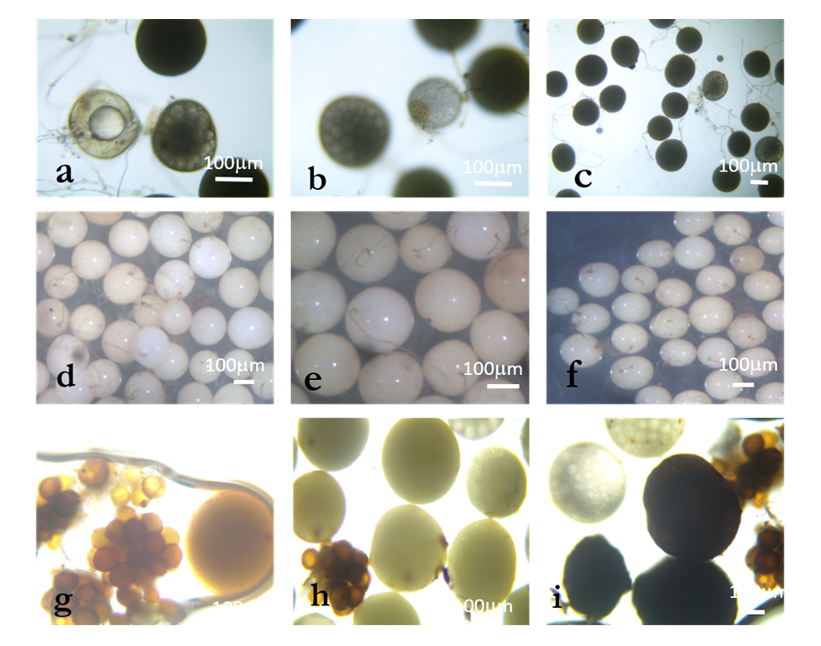 | Figure 2. Spores of the characterized morphotypes; a, b and c: Acaulospora; d, e and f: Gigaspora; g, h and i: Glomus |
|
|
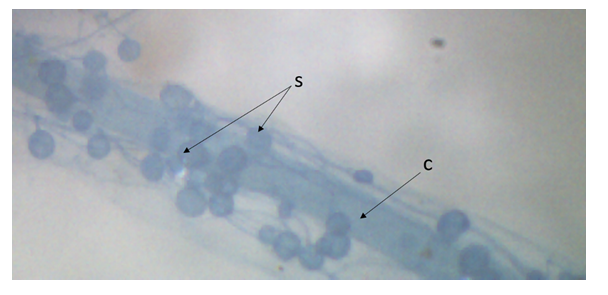 | Figure 4. Colored roots of Sorghum bicolor plants after trapping (s = Intra-root spores, c= root cortex) |
|
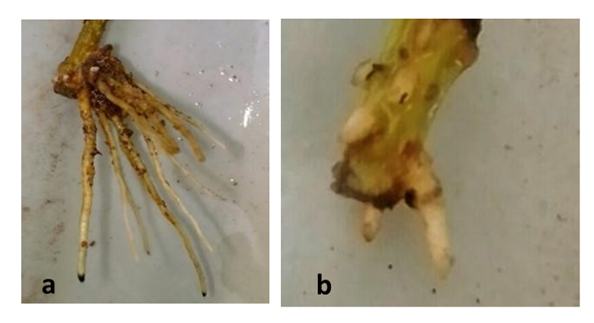 | Figure 5. Prunus cuttings, a: root system of the inoculated cuttings; b: root system of un-inoculated cuttings |
4. Discussion
- Isolation and characterization of Arbuscular Mycorrhizal FungiThe number of morphotypes recorded in the Mount Cameroon (9), Mount Oku (6) and Mount Manengouba (4) sites has reflected the diversity of these sites. These results did not match with those obtained by Nzweundji and al. (2015) in the same sites, which at that time had fewer morphotypes (6). This difference could come from the fact that the fungi present in these sites have adapted and evolved. Over time, the environment would have become favorable to accommodate new species according to the variations in soil composition influenced by the growth of different plant species (Ngonkeu, 2009).The morphological identification carried out in this study made it possible to determine the different families of mycorrhizal fungi colonizing the roots of P. africana which were found to be: Gigaspora margarita (Gigasporaceae), Acaulospora sp (Acaulosporaceae) and Glomus aggregatum. These species were characterized using morphological characters: the shape, size, color and external appearance of the spore. The morphological identification has many limitations (Schüßle et al., 2001; Zeramdini, 2009) but the results obtained were in line with those of Tchiechoua et al., (2015). As mycorrhizal fungi are obligatory symbionts, to ensure their symbiotic efficiency, the Arbuscular Mycorrhizal fungi spores identified were previously pre-germinated on an agar medium. This technique made it possible to have almost all spores germinated with young mycelial filaments active in the formation of mycorrhizal associations. This result is similar to that obtained by Ngonkeu (2009), in the same culture substrate.Evaluation of the efficiency of Arbuscular Mycorrhizal fungi on the vegetative growth of Prunus africana cuttingsSpores are organs for the perpetuation of species. Under favorable conditions, these spores establish symbiotic (mycorrhizal) relationships with plant roots for mutual growth. When these symbioses break up, Arbuscular mycorrhizal species remain thanks to sporulation. This principle has been highlighted in this study. The results of the multiplication of mycorrhizal fungi from a single spore and from several spores have shown that these beings multiply and colonize their living environment regardless of their starting number. Functional diversity can be determined through an assessment of the variability in plant response to mycorrhizal inoculation. This was significantly recorded after inoculation of 6 AM fungi strains on Sorghum plants in a greenhouse. Morphologically identical spores introduced into culture gave an identical response on the growth and development of sorghum plants, thus demonstrate good identification. This result showed a slight difference with Ngonkeu's work (2009), which was probably due to a difference in precision during the morphological characterization. Analysis of the data showed that there was no significant difference in growth of plants inoculated with one spore and that with several spores, in accordance with the work by Jansa et al., (2008) and Olfat and Jalil (2012) on root infections and multiplication of spores. This could be explained by the fact that the establishment of a mycorrhizal symbiosis depends on the quality of the information transmitted by the partners present, the information being transmitted by Strigolactone for the plant to induce a Mycfoctor response in the Fungi. This exchange is made independently of the amount of AM fungi in the medium or substrate (Jalil and Olfat 2012).The rooting capacity of P. africana cuttings can be improved by growth hormone (Avana, 2006). The results of this study showed that the contribution of indole butyric acid (AIB) treatment stimulated the rooting of P. africana cuttings, but mycorrhizal fungi induced a more pronounced development of root system. This can be explained by the fact that mycorrhizal fungi, by intensifying the nutrient absorption surface of the plant partner, protect them against root pathogens, stimulate the production of their natural phytohormones (essential for rooting) and contribute to the increase of their active substances (Augé, 2004). In return they receive their carbon nutrients from the plants (Ludwig-Müller and Güther, 2007). Hence, the contribution of the latter becomes incomparable to any other exogenous contribution, in line with the work by Veresoglou et al., (2012) on the impact of AM fungi on root development, nutrition and protection of plant cuttings.
5. Conclusions
- The general objective of this work was to assess the efficiency of native AMF species in the improvement of the growth of root cuttings to ameliorate the regeneration of P. africana. From this work, six species of AM fungi belonging to Gigaspora, Glomus and Acaulospora genera were identified to be dominant and active in the rhizosphere of P. africana in Cameroon. Among the species, Gigaspora margarita was found to be the best arbuscular mycorrhizal in the improvement of Prunus africana cuttings development. Their effects on root system development were more pronounced compare to those of plant growth hormone AIB. The use of these particular species in the vegetative regeneration of Prunus Africana, can improve their root system density and enable cuttings to effectively resist field transplantation. This mycorrhizal symbiosis could represent an innovative approach to solve the problem of Prunus africana regeneration.
ACKNOWLEDGEMENTS
- The authors are thankful to the University of Yaoundé 1 and the Institute of Agricultural Research for Development (IRAD) for the facilities they provided for the implementation of this work. They also thank the International Foundation for Science (IFS) for funding every single step of the work.
Funding
- International Foundation for Science (IFS) (grant number D/5823-1).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML