-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 10(3): 45-52
doi:10.5923/j.plant.20201003.01

Morphological Characterisation and Variability Analysis of African Yam Bean (Sphenostylis stenocarpa Hochst. ex. A. Rich) Harms
Ademola I. Aina1, 2, Christopher O. Ilori1, Ukoabasi O. Ekanem2, Olaniyi Oyatomi2, Daniel Potter3, Michael T. Abberton2
1Department of Crop Protection and Environmental Biology, University of Ibadan, Ibadan, Nigeria
2Genetic Resources Centre, International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria
3Department of Plant Sciences, University of California, Davis, U.S.A
Correspondence to: Ademola I. Aina, Department of Crop Protection and Environmental Biology, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

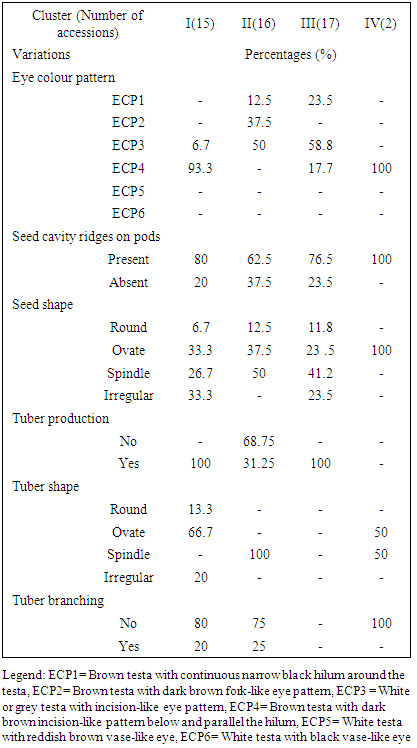
African yam bean (Sphenostylis stenocarpa) is a legume that produces tubers rich in protein and with potential to contribute to food and nutrition security in tropical Africa. African yam bean (AYB) is underused despite its nutritional profile. Knowledge of the genetic diversity present in AYB germplasm would be beneficial to the utilisation of its genetic resources in the improvement of the crop. This study aims to understand the genetic diversity in a collection of 50 accessions of AYB. Thirty morphological and taxonomic phenotypic traits of high descriptive value were characterized and the levels of diversity among the accessions were assessed using analysis of variance (ANOVA), Correlation, Principal Components, and Clustering analysis. The 50 accessions were grouped into four clusters of 2-17 members at a dissimilarity index of 0.7. Out of 30 morphological variables analysed, eye colour pattern was the most discriminatory and it classified the accessions in the proportion of 38% with white or grey testa with incision-like eye pattern and 62% with brown testa and varying patterns from narrow stripe to fork-like or incision-like eye pattern around the hilum. The stepwise discriminant and frequency procedures in SAS also identified other phenotypic variables with significant discriminatory attributes. Accessions with desirable agronomic characters such as lower number of days to flowering, high number of pods per plant and total seed weight per plant were also identified in this study.
Keywords: African yam bean, Genetic diversity, Accessions, Phenotypic traits
Cite this paper: Ademola I. Aina, Christopher O. Ilori, Ukoabasi O. Ekanem, Olaniyi Oyatomi, Daniel Potter, Michael T. Abberton, Morphological Characterisation and Variability Analysis of African Yam Bean (Sphenostylis stenocarpa Hochst. ex. A. Rich) Harms, International Journal of Plant Research, Vol. 10 No. 3, 2020, pp. 45-52. doi: 10.5923/j.plant.20201003.01.
Article Outline
1. Introduction
- In sub-Saharan Africa, increasing food options through effective utilisation of neglected and underused crops will help to reduce hidden hunger, environmental and income vulnerability in the face of unpredictable weather conditions [36]. African yam bean (Sphenostylis sternocarpa Hochst. ex A. Rich.) Harms) is an underutilised legume with potential to address the food and nutritional security issues of tropical Africa. African yam bean (AYB) produces edible tubers and seeds and is considered the most economically important species in the genus Sphenostylis [27,28]. This neglected legume [29,18] has been an important traditional crop in Africa with several sociocultural uses [2]. For instance, the crop is considered a significant substitute for cowpea (Vigna unguiculata (L.) Walp.) during lean periods in the eastern parts of Nigeria [23]. Also, the lectin found in AYB seeds has been reported to be effective in the control of some leguminous pests [24,22]. African yam bean has a good amino acid profile, containing more lysine and methionine content than is found in pigeon pea (Cajanus cajan (L.) Millsp.), cowpea and Bambara groundnut [33]. Its grain and tuber contain approximately 29% and 19% crude protein, respectively [2]. In West Africa, particularly Nigeria and Ghana, only few aged farmers and old market women still hold and use some available landraces [6,30], hence genetic erosion is imminent as the few landraces may be lost with the death of the farmers. Conservation ex-situ or in situ in genebanks is the only approach to preserve the genetic resources of species producing orthodox seeds [8]. The commercial exploitation of AYB will rely on superior and improved varieties. The continual collection and assessment of the diversity present in landraces will enhance the success of breeding for hybrid varieties. Diversity analysis of AYB has been done using morphological [4,26,2,21] and molecular characterisation [16,3,20,31]. The Genetic Resources Centre of the International Institute of Tropical Agriculture has been carrying out timely collection of AYB for conservation. Morphological characterisation precedes selection for genetic variability and identification of promising accessions with desirable traits of interest. This present study aims to assess the genetic diversity in 50 accessions of AYB and to identify discriminatory phenotypic variables in AYB.
2. Materials and Methods
2.1. Plant Materials and Experimental Lay Out
- Fifty accessions of African yam bean (AYB) were obtained from the Genetic Resources Centre, International Institute of Tropical Agriculture (IITA), Ibadan. The passport data of the selected accessions are presented in (Table S1). Two to three seeds of each accession were sown on double row plots of 5m long each with an intra and inter-row spacing of 1m apart. Seedlings were thinned to one plant per stand after emergence. The experiment was performed during the cropping seasons of 2015/16 and 2016/17 (August to February). Three weeks after planting, the seedlings were staked and carefully twined in a clockwise manner around the stakes. Insects were controlled with a mixture of Paraeforce and Cyperdiforce at the rate of 35-60 mL in 20 litres of water every 2 weeks during the flowering period. Weeding and other field maintenance practices were performed until maturity. The experiment was laid out in a randomised complete block design and replicated three times. Data on morphological variables were taken and recorded from five representative plants of each accession on the two plots, using the IITA descriptor list for AYB [1].
2.2. Data Analysis
- The Gower distances [9] for continuous, ordinal and binary variables were calculated and cluster analysis of the AYB accessions using Ward minimum variance method [34]. Analysis of variance (ANOVA) and Stepwise discriminant approach [10] were applied on the continuous and log transformed ordinal variables to determine the critical variables on the clustering groups. The step discriminant model identifies variables in a sequential manner, starting with the highest F value from an ANOVA and explores the possibility of any relationship between the observed variables. A maximum P value of 0.15 was employed in assigning a new variable in a set of significant variables, as recommended by [10]. Principal component analysis was calculated to determine the interrelationship among morphological variables using PRINCOMP procedure in SAS-V9 [37]. The relationship between pairs of morphological traits was calculated using Pearson’s correlation coefficient [25]. ANOVA, Cluster analysis, Step Discriminant analysis was performed using CLUSTER, GLM and STEPDISC from the Statistical analysis system, SAS-V9.3 [37].
 | Plate 1. AYB plants growing in the field |
3. Results and Discussion
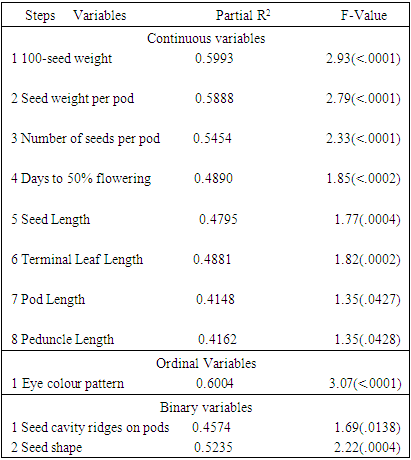
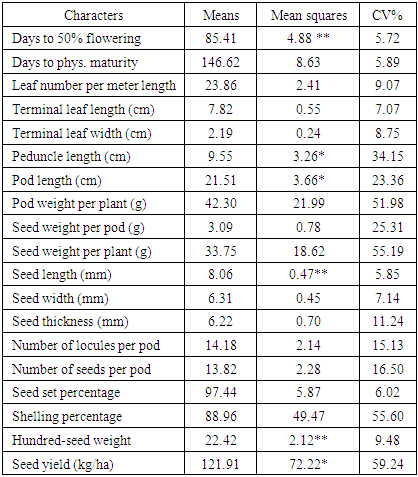
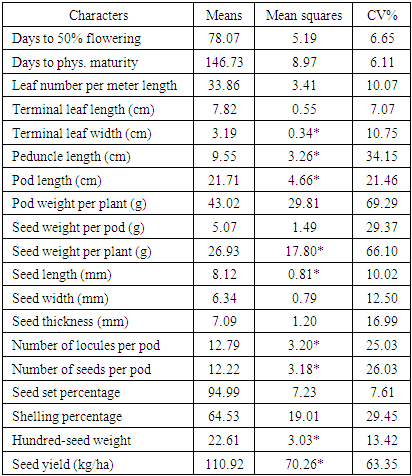
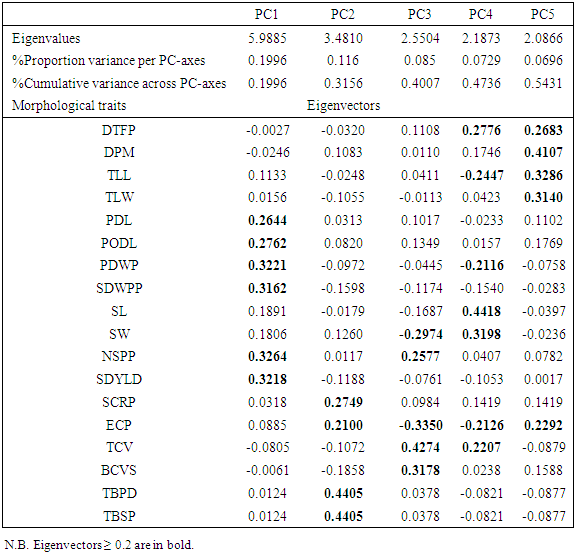
- Most (80%) of the accessions studied here were collected from different parts of Nigeria. The rest 20% had not available passport data. A total of 30 variables were used in descriptive statistics for the accessions studied. Twenty-two continuous variables were recorded while the eight ordinal variables were all seed and tuber related. The variables showed variation in their strength for distinguishing the 50 accessions. For instance, the pod length was about six times the length of the shorter ones (Table S1) and days to physiological maturity varied considerably from 132 to 165 days after planting. The most discriminative ordinal variable (P ≤ 0.0001) was the eye colour pattern (Table 1) while seed shape and cavity ridges were the most discriminatory binary variables (P ≤ 0.0138). These variables in descending order of; 100-seed weight, seed weight per pod, number of seeds per pod, days to fifty per cent flowering, seed length, terminal leaf length, pod length and peduncle length were the most significant continuous variables (P ≤ 0.0427).
|
|
|
|
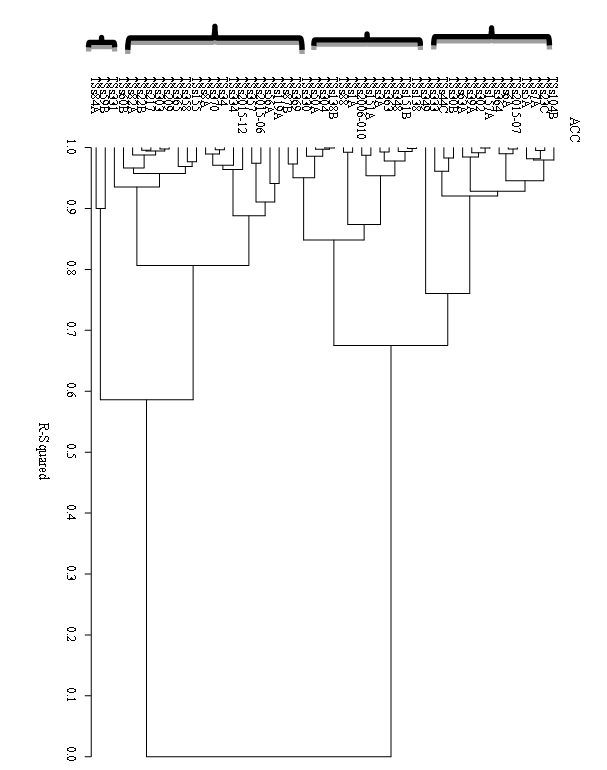 | Figure 1. Dendrogram showing the clustering pattern of 50 African yam bean accessions |
4. Conclusions
- To improve AYB yield, number of seeds per pod, number of pods per plant, weight of seeds per pod and weight of seeds per plant are important factors to be considered in breeding programmes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML