-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 10(2): 40-43
doi:10.5923/j.plant.20201002.03

Effect of Saline Toxicity Solutions on the Growth Performance of Bambara Groundnut (Vigna subterranea L. Verde)
Barka Peter Mshelmbula1, Solomon Peter Wante2, Sulaiman Bello1, Joel Osajie1
1Department of Plant Science and Biotechnology, Federal University Lafia, Nigeria
2Department of Biological Sciences, Federal University of Kashere, Nigeria
Correspondence to: Solomon Peter Wante, Department of Biological Sciences, Federal University of Kashere, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

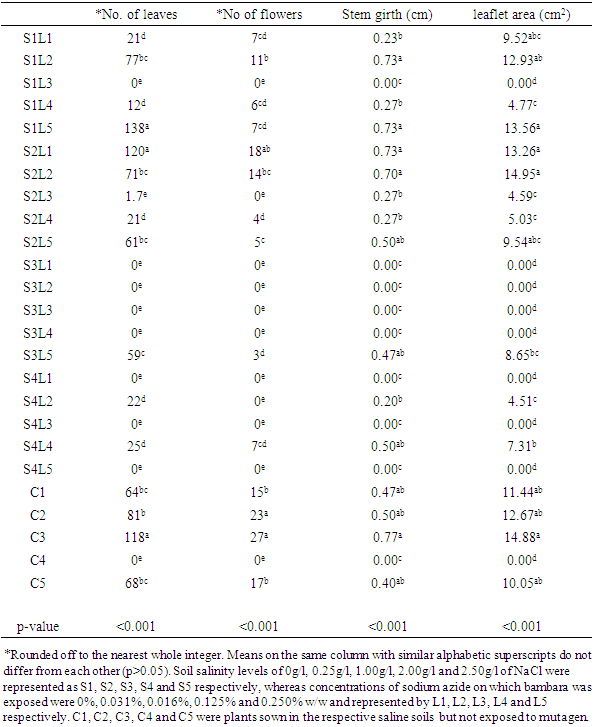
The Bambara groundnut (Vigna subterrenea (L.) Verde) is an important source of affordable proteins from a vegetable legume in sub-Saharan Africa. Although, the percentage content of amino acids found does not meet the standard recommendation specified by FAO/WHO due to the limited amount of tryptophan. Protein intakes are essential to satisfy the metabolic needs of amino acids and nitrogen balance in humans. The ability of bambara groundnut to survive and grow under harsh environmental conditions has an under turn specific adaptive traits which includes morphological and physiological adaptions. Salinity toxicity is one of the major limiting factors in crop production worldwide. The deleterious effect of saline toxicity depends on the type of plant species. Therefore, here in this study, various concentrations of saline toxicity solutions of 0.25g/l, 1.0g/l, 2.0g/l and 2.5g/l were evaluated in relation to the growth performance of Bambara groundnut. For example, there were as least as 20 leaves in S1L1, compared to S1L5 which had as much as 138 leaves per plant. The toxicity can either be due to Na+ or Cl- ion which can rapidly build up in the cytoplasm and inhibits enzyme activity or the build-up in the cell walls and dehydrate the cell.
Keywords: Bambara groundnut, Salinity, Protein and Toxicity
Cite this paper: Barka Peter Mshelmbula, Solomon Peter Wante, Sulaiman Bello, Joel Osajie, Effect of Saline Toxicity Solutions on the Growth Performance of Bambara Groundnut (Vigna subterranea L. Verde), International Journal of Plant Research, Vol. 10 No. 2, 2020, pp. 40-43. doi: 10.5923/j.plant.20201002.03.
1. Introduction
- Bambara groundnut (Vigna subterrenea (L.) Verde) is a major source of vegetable protein in sub-Saharan Africa. It is also an important source of affordable proteins in the diets compare with the animal source of protein [1]. The crop is richer in essential amino acids than other legumes, for example, it has a protein content of 6% and 10% higher than soya bean and cowpea, respectively [2]. Although, the percentage content of amino acids found does not meet the standard recommendation specified by FAO/WHO due to the limited amount of tryptophan [3]. Protein intakes are essential to satisfy the metabolic needs of amino acids and nitrogen balance in humans [3].Despite the nutritional value of bambara groundnut, it has not received much research attention compared to other legumes [4]. The plant has been grown under harsh environmental conditions with relatively low labour inputs during cropping. The ability of bambara groundnut to survive and grow under harsh environmental conditions is related to specific adaptive traits which include the production of subterranea pods [5]. Physiological adaptation includes the regulations of osmotic adjustment, leaf area index reduction, an increase in leaf reflectivity, and changes in leaf orientation and stomata regulation [6]. Salinity toxicity is one of the major limiting factors in crop production worldwide [7]. Salinity reduces plants growth through osmotic stress, specific-ion toxicities nutritional imbalances [8]. Although the timing of the deleterious effect of the saline toxicity depends on the type of plant species [8]. Therefore, it was of significance to evaluate the growth performance of Bambara groundnut (Vigna subterrenea (L.) Verde) to saline toxicity solutions.
2. Results and Discussion
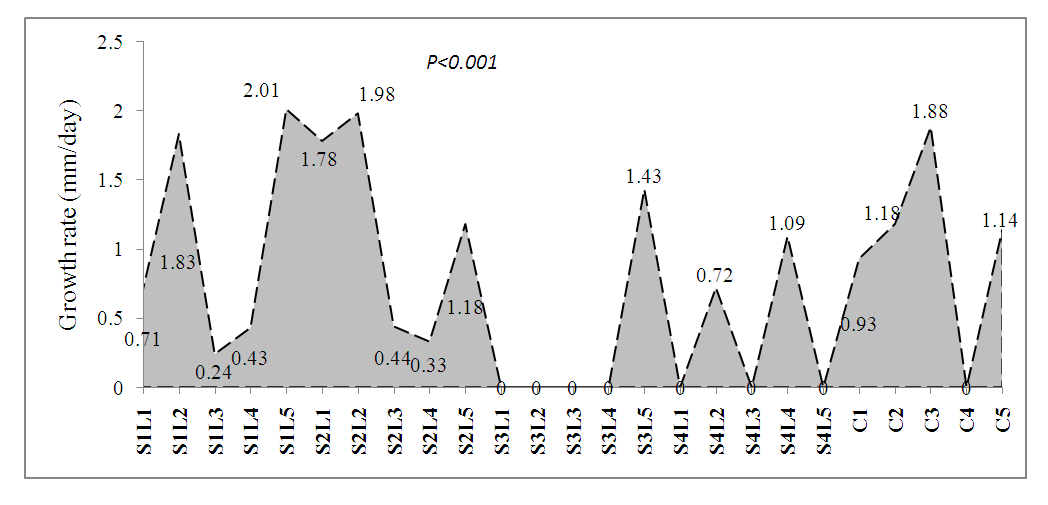
- In the present study, salinity conditions were created, by saturating 10 kg air-dried soil with 0.25g/l, 1.0g/l, 2.0g/l and 2.5g/l of NaCl solution respectively. Prior to sowing, viable seeds of bambara groundnut were soaked in 0.031%, 0.016%, 0.125% and 0.250% w/w NaN3 solution for 6 hours. The treated seeds were rinsed in running water to remove excess chemicals and exudates from seeds and then sown on designated experiment buckets bearing each salinity level. The growth rate of the test plant was calculated every day as the daily millimetric increase in height of plant (Fig. 1). Results showed that plant treatment with the highest growth rate was S2L2 with a rate of 1.98mm/day, followed by C3 (1.88 mm/day). Plants in 1.0g/l salinity levels generally did not show any further increase in growth.
|
3. Conclusions
- In conclusion, the present findings have contributed to the understanding of salinity toxicity on the growth performance of Bambara groundnut (Vigna subterrenea (L.) Verde). From the results of this study, we can interpret that the salinity toxicity concentrations alone have not caused much of the detrimental damage to the growth parameters of the bambara groundnut plant compared with the combined effect of the NaCl with sodium azide concentrations. Therefore, we presumed that combination of NaCl with sodium azide concentrations may have formed optimal concentrations enough to damage the physiological properties of the bambara groundnut seedlings which may have altered the cell plasma membrane to allowed influx or efflux of ions. There is substantial evidence from this study that combined effect NaCl with sodium azide concentrations has induced morphological developmental parameters of the bambara groundnut plants. For example, S1L5 had as much as 138 leaves per plant compare with S2L3 which has fewer than 2 leaves.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML