-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 10(1): 17-26
doi:10.5923/j.plant.20201001.03

Effects of Waterlogging on the Growth and Chlorophyll Content of Ixora coccinea Lin. (Jungle Flame)
Oluwole Surukite O.1, Ogun Mautin L.1, Arowosegbe Sunday2, Osonubi Oluwole3, Osinaike Titilola S.1, Sanni Adetayo A.1
1Department of Botany, Lagos State University, Ojo, Nigeria
2Department of Plant Science and Biotechnology, Ekiti State University, Ado-Ekiti, Nigeria
3Department of Botany, University of Ibadan, Oyo State, Nigeria
Correspondence to: Ogun Mautin L., Department of Botany, Lagos State University, Ojo, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Effects of waterlogging on the growth and chlorophyll content of seedlings of two cultivars of Ixoracoccinea (Red and Yellow) were carried out on one hundred seedlings of Ixora. Waterlogging experiments viz: Waterlog Treated plants (WTP) were subjected to constant waterlogging at 5cm above the soil level while Control (CTR) were watered with 35cl of water daily. Data on the growth parameters and chlorophyll contents of the two cultivars of Ixoracoccinea were collected for 10 months. All the data collected were subjected to Analysis of Variance (One way ANOVA) at P<0.05. The results obtained showed that both Red and Yellow Ixora CTR and Red Ixora WTP had similar performances in number of leaves, stem height, leaf area and leaf area ratio and fresh biomass except chlorophyll content. The results however revealed that Yellow Ixora seedlings in waterlog and control performed better than those of Red Ixora seedlings morphologically. As regards the chlorophyll content, Red Ixora showed higher chlorophyll content against the Yellow Ixora; Red Ixora Control and Waterlog Treated Plants showed no difference in their chlorophyll contents. Yellow Ixora Control showed higher chlorophyll content compared to the Waterlog Treated Ixora ones. Thus, from the study, it could be concluded that Ixoracoccinea (Red and Yellow) plants are waterlog tolerant plants with 100% survival rate. However, Yellow Ixora cultivar grew better than Red Ixora, and should be considered first for the beautification and improvement of floras of flood prone areas (Lagos State).
Keywords: Waterlogging, Morphology, Growth, Lenticels, Chlorophyll content, Ixora coccinea
Cite this paper: Oluwole Surukite O., Ogun Mautin L., Arowosegbe Sunday, Osonubi Oluwole, Osinaike Titilola S., Sanni Adetayo A., Effects of Waterlogging on the Growth and Chlorophyll Content of Ixora coccinea Lin. (Jungle Flame), International Journal of Plant Research, Vol. 10 No. 1, 2020, pp. 17-26. doi: 10.5923/j.plant.20201001.03.
Article Outline
1. Introduction
- Without any iota of doubt, the last 100 years has faced unbridled exploitation of the world’s natural resources (land and water) and this has resulted in severe damage to its vegetation including accumulation of industrial wastes and greenhouse gases. Together, these have upset natural ecosystem balances and have created many environmental and climatic problems, including rising temperatures, increasing desertification, serious soil loss, and increased flooding [1,2,3]. In many nations, the recent increased flooding are coming into sharp focus because of their sudden, long term and devastating consequences for plant, animal and human populations [4].Waterlogging is a common environmental stress and natural phenomena such as rainfall; snowmelt, tides and human activities such as the construction of tidal water conservancy and hydropower can result in flood-prone environment [5,6]. Waterlogging alters the original growth environment and conditions experienced by plants thereby greatly affecting plant growth and physiological rhythms. The reduction in oxygen available for roots as a result of flooding is a major factor restricting plants survival, growth and development [7]. The effects of waterlogging on plants includes inhibition of growth of roots, shoots and new leaves and in turn causing decreased growth in the entire plant; reductions in the net photosynthetic rate, photosynthetic electron transport rate and photo-system II (PS II), photochemical efficiency [8,9]; reactive oxygen species (ROS) metabolism disorders [10], reductions in element uptake; and inhibition of transport from roots to leaves [11]. However, a wide variety of plants are known for the tolerance to water stress and oxygen deficiency during the adult stages of their life cycle [12,13]. All plants have tolerance to water stress, but the extent varies from species to species [12]. For example, flood sensitive plants like Lycopersicum esculentum, Glycine max, and Helianthus annuus are killed in the waterlogged conditions, while plants like Oryza sativa can withstand water-logging for a considerable time. However, continuous submergence of Oryza sativa is also deleterious resulting in death and decay of the plant [14]. Rapid change in soil properties takes place following soil water-logging. As water saturates the soil pores, gases are displaced; a reduction in gas diffusion occurs and phyto-toxic compounds accumulate as anaerobic condition prevailed. Water stress in plants reduces the plant cell water potential and turgor, which elevate the solute concentration in the cytosol and extracellular matrices. As a result, cell enlargement decreases leading to growth inhibition and reproductive failure. This is followed by accumulation of Abscissic acid (ABA) and compatible osmolytes like proline which causes wilting [15,16], cellular damage, light imbalance and denaturing of functional and structural macromolecules [17,18].However, some plant species can sense low oxygen level via the post-translational regulation of key pathway to enhance plant responses to hypoxia and flooding [19,20,21]. This brings about various morphological, physiological and biological changes that improve flood tolerance [22,23]. These changes include alteration of the light absorption efficiency of plants through changes in leaf morphology with a reduction of light absorptions [24], the formation of adventitious roots and hypertrophic lenticels at the stem base [25], the reopening of stomata [26]; the formation of a complex antioxidant defense system [27] and reductions in carbohydrate consumption and changes in nutrient partitioning [28]. Growing plants in wet, poorly drained soil can be quite difficult. To the extreme, very heavy rainfall followed by flooding (waterlogging) cannot cause only tremendous damage to buildings and homes, but also can kill woody and herbaceous plants, while some other plants remain unaffected [29].Since plant (crop) production is affected by various environmental factors including both biotic and abiotic factors. Water availability is one of the essential abiotic factors which might affect plant's growth through altering the function of plant roots and soil borne microbes such as root endophytic fungi, mycorrhizal fungi, rhizobia, and plant growth-promoting microorganisms [30]. To alter plant performance through the interaction between plant roots and soils, several elements such as water availability, soil type, and nature of plant may be involved. It would depend on the time of the year the flood event occurs, duration of the flood event, species sensitivity to flooding and the type of soil the plants are grown. Dominant plants are more tolerant to waterlogging than active growing plants. For example, Jull [27] reported that Avicennia and Mangroves survived longer in flooding than actively growing plants (mesophytes such as Almond and Mango plants) to flooding.Thus, it becomes important for botanists to identify diverse taxa of trees that would withstand highly variable soil moisture conditions in urban areas. Both deficiencies and excesses in soil moisture contribute to decline of city trees [9,31] and conservation of water amid continued urbanization is being promoted through annual tree planting programs. Although the use of numerous species resistant to waterlogging is needed to prevent monocultures prone to destruction by pests and diseases, reports has shown that only seven species accounted for 75% of the street trees planted in the United States in 1980 but today several species have been identified and more needed to be identified. Examples of some of the plants that have been identified include Distylium chinense, Erythrina speciosa, Annona glabra, Tabebuia avellanedae, Genipa americana. These plants have been found to adjust to waterlogging by creating decrease in whole plant biomass and root biomass; production of adventitious roots, hypertrophic lenticels, and so on [9]. Thus, Ixora coccinea is a plant with likeable potential of adjusting to waterlogging.Ixora coccinea Lin. is a tropical to semi- tropical evergreen shrub belonging to the Rubiaceae family. It is the only genus in the tribe Ixoreae. It consists of tropical evergreen trees and shrubs and holds around 545 species [32]. Though, native to the tropical and subtropical areas throughout the world, its centre of diversity is in tropical Asia. This is ideal for solid earth embankment due to its strong root system [33]. The plant is of a strong horticultural value owing to its large flower clusters which comes in red, orange, yellow and pink based on varieties. Thus, common names are Jungle flame and Flame of the wood. Its flowers, leaves, roots and stem are also used to treat various ailments in the Indian traditional system – (Ayurveda) and in various folk medicines. The fruits when fully ripe are used as a dietary source. Bonnie [32] demonstrated that I. coccinea have blooms of clusters of petaled floret which last for four to six weeks on the stem in a well-drained and moderately acidic soil. However, little is known about the morphological and physiological responses of I. coccinea to waterlogging. Thus, this study tends to determine the response of I. coccinea to waterlogging, thereby enhancing beautification; thus, providing a theoretical basis for vegetation screening and recovery of the hydro-fluctuation (flood prone areas) zones like Lagos.
2. Materials and Methods
- Collection of MaterialsExperiments were carried out in a screenhouse located at Botanical Garden of the Department of Botany, Faculty of Science, Lagos State University, Ojo- Lagos, Nigeria for the period of 10 months. One hundred seedlings of two cultivars of Ixora coccinea (50 Red single syn “Maui” and 50 Yellow single syn “Maui Yellow seedlings each) of about 5 months old were obtained from Gabby Modern Eden Horticultural Garden along Lagos State University (LASU) - Igando Road, Officers Village Bus-Stop, Ojo- Lagos State. The Poultry manure was collected from a Poultry farm in Ojo, Lagos; and loamy soil was obtained from the Botanical garden of Lagos State University.Soil Preparation Loamy soil collected from the Botanical garden was thoroughly mixed with poultry manure; 5kg of the mixture were measured using weighing balance into equally perforated plastic pots of (22 cm in depth and 16.5 cm in diameter). At the end, a total of 100 (one hundred) pots were prepared and watered lightly for 7 days to enable the decomposition of the organic manure to prevent shock and suffocation to the seedlings.Seedling Transplant and EstablishmentThe one hundred seedlings of Ixora (Red and Yellow) obtained was transplanted. The seedlings were allowed to establish itself in the new environment for 3 months with light irrigation daily.Waterlogging Experiment/Treatment InducementThe established seedlings of Ixora coccinea L cultivars- Red and Yellow were divided into two groups [Waterlog Treated Plant (WTP) and Control (CTR)]. The Red and Yellow Ixora coccinea cultivars comprised of 50 seedlings for Waterlog Treated plant (WTP) and 50 seedlings for control respectively. The Waterlog Treated Plants (seedlings) were placed in a bowl of (12cm in depth and 30cm in diameter) containing 3 litres of water while the control seedlings were not. The WTP were watered up to 5cm above the soil level three times daily to maintain constant flooding of the seedlings while the control treated seedlings were watered with 35cl of water using measuring cylinder once daily to prevent flooding. A complete randomized designed was used for this experiment.Harvesting/Morphological Data CollectionThe first harvest of the fresh seedlings were collected from 3 replicates per treatment after carefully removed from the watered soil to prevent loosing the roots and washed with clean water to remove particles at the end of the third month of seedlings’ establishment (90 days after seedling transplant) for initial morphological data and subsequently, plants (WTP and CTR) were harvested at monthly intervals until when the experiment was terminated after the seventh month (210 days) after inducement of treatment. The morphological characters measured are Number of Leaves (NL), Stem Height (SH), Root Length (RL), Fresh Root Weight (FRW), Fresh Leaf Weight (FLW), and Fresh Stem Weight (FSW), Leaf area (LA) and Leaf Area Ratio (LAR).The stem height, and length of roots were measured in centimeters using meter rule, the plant girth were measured in centimeters using vernier caliper while fresh and dry weights of root, stem and leaf were measured using electronic balance. Thus, the Leaf Area (cm2) and leaf area (cm2/g) ratio was calculated using the formula of Okubena-Dipeolu et al. [34]:Leaf area (cm2) = 0.853+ (leaf blade length* leaf blade breadth)* 8.7440 Leaf Area Ratio (cm2/g) = Leaf Area (cm2)/Total Dry weight (g).The dry weight of the roots, stems and leaves were obtained by placing the respective plant parts in the oven at 80°C for one day at Botany Laboratory of Lagos State University, Ojo, Lagos, Nigeria. Determination of Leaf Chlorophyll ContentThe chlorophyll content of the Ixora coccinea leaves were estimated using Hipkins and Beaker [35], Dunn et al. [36] and Sumanta, et al.’s [37] methods with slight modification. Freshly excised 0.5g leaves of Ixora coccinea L (upper, middle and lower on the main branches) were weighed. The sample were chopped and transferred into 20ml of 98% Di-methyl sulfoxide (DMSO). The mixture was stored in the refrigerator (10°C) until the last month of experiment for one time analysis. The absorbance of the extracts was then read in visible spectrophotometer (model V5000) at 665 and 649 wavelengths. The measurements were replicated thrice using leaves from three different parts of different plants for each treatment. The chlorophyll content (mg l-1) was calculated using the following equations according to Ekanayake et al. [38] and Sumanta et al. [37].Chlorophyll a (Chla) = 12.47*A665- 3.62*A649; Chlorophyll b (Chlb) = 25.06*A649. – 6.5*A665Total Chlorophyll (Chl) = 20.2*A649 + 8.02*A665Where A = Absorbance.Statistical Analysis The data obtained from the study for various plant parameters was subjected to single univariate summary statistics such as the mean and standard deviation. The analysis of variance (ANOVA) was then used to compare the variability in the selected parameters due to the treatment application with the aid of the software MITAB 2007 version 15. Significant means were separated by Least Significance Difference test (LSD) at the 95% probability level using Fisher Pair-wise Comparisons, according to procedure outline by Metwally et al. [39].
3. Results
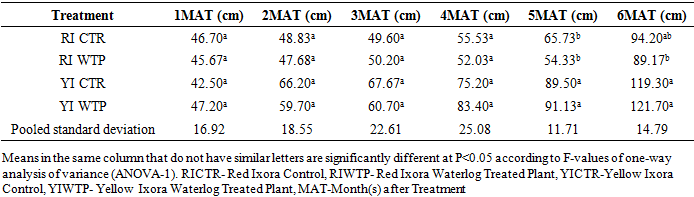
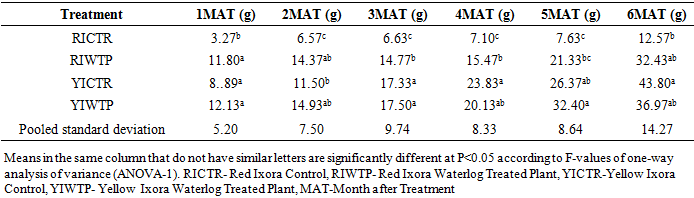
- Effects of Waterlogging on Morphological charactersStem heightThe result on the effects of waterlogging on stem height of Ixora coccinea showed no significant differences at P<0.05 between the Red Ixora Control (RI CTR) and Red Ixora Waterlog Treated Plants (RI WTP) from the first month after treatment to the end of the experiment (Table 1). Also, there were no significance differences in the stem heights of Yellow Ixora Control (YI CTR) and Yellow Ixora Waterlog Treated Plants (YI WTP) (Table 1).The results however revealed that Yellow Ixora plants (YI CTR and YI WTP) showed significantly greater stem heights (P< 0.05) than Red Ixora cultivar (RI CTR and RI WTP) from fifth month after treatment to the end of the experimental period (Table 1).
|
|
|
|
|
|
|
|
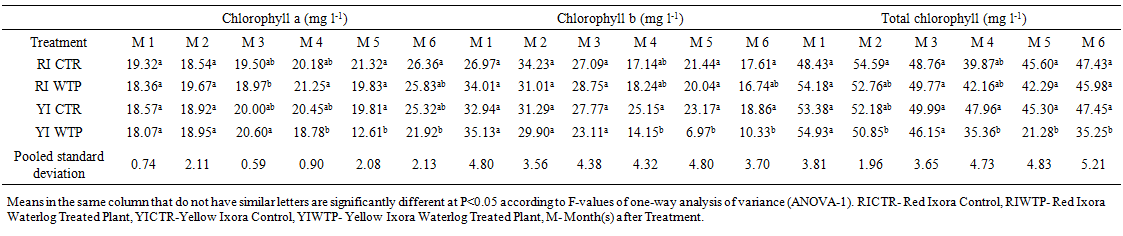 | Table 9. Effects of Waterlogging on the Chlorophyll content of Ixora coccinea |
4. Discussion
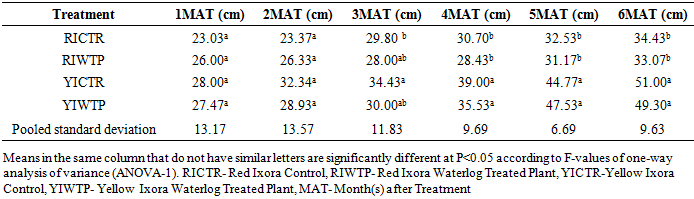
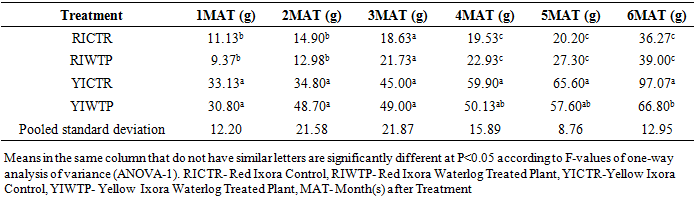
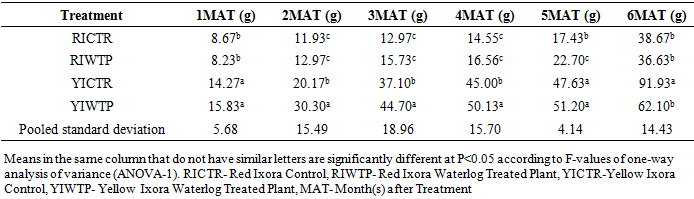
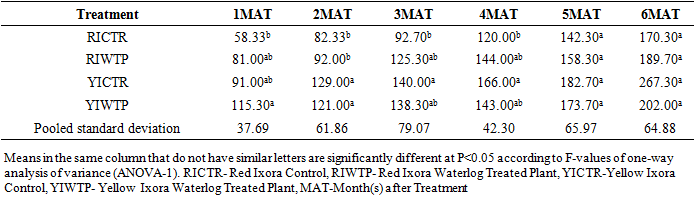
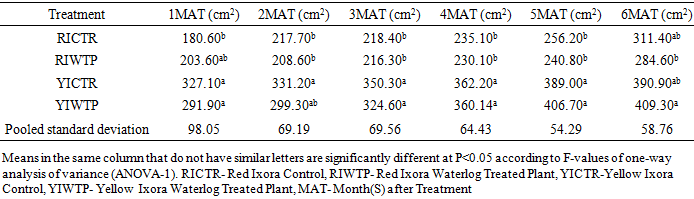
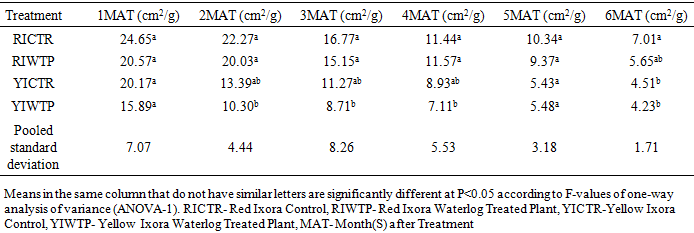
- Effects of Waterlogging on the Morphological Growth CharactersIn plants generally, an appropriate growth strategy is key to fitness in a threatening and or competitive situation, so too in Ixora coccinea seedlings, their growth strategy is critical to survival [40]. The response (morphological characters) of Ixora coccinea exposed to Waterlogging (Tables 1-8, P<0.05), revealed a contrasting results. The results highlight the high tolerance of two I. coccinea cultivars, as indicated by 100% survival rate and only slight differences compared to the control and across the two cultivars (Red and Yellow Ixora). Similar results have been reported by Peng et al. [41], who found out that the survival rate of Distylium chinense seedlings was 100% when faced with 30 days summer flooding, with no symptoms of severe injury. Furthermore, several studies on the effects of flooding in woody plants have demonstrated adjustments in morphological characters such as root length, stem height, leaf length and so on contribute to flood tolerance [42,43,9]. In the present study, the results showed that Waterlogging had great significant effects on stem height, leaf area and leaf area ratio, root length and number of leaves in Red Ixora (CTR and WTP) compared to Yellow Ixora (CTR and WTP) (Tables 1-8). This was because hypertrophic lenticels and abundant adventitious roots were produced on the submerged portions of the stems, which improves oxygen diffusion from aerated parts to the root system (Tables 2). This finding agreed with works of De Olivera and Joly [7] and Liu et al. [9], when they worked on the Flooding tolerance of Calophyllum brasiliense Camb: morphology, physiological and growth responses; and Effects of off-season flooding on growth, photosynthesis, carbohydrate portioning and nutrient uptake in Dystylium chinense respectively. However, it was observed that, growth performance between Red Ixora Waterlog Treated Plants and Red Ixora Control Treated Plants; as well as Yellow Ixora Waterlog Treated Plants and Yellow Ixora Control Treated Plants were indifferent. Notably, Yellow Ixora cultivar grows faster and taller than the Red Ixora cultivar (Tables 1-2, 6-8). This was also supported by Metwally, et al. [39], that reported the inhibition of plant growth characters and flowers yield under water stress may be due to exposure to injurious level of flood causing a decrease of turgor which resulted in a decrease of growth and development of cells, especially in stems and leaves. Also, Oluwole et al. [44], said reduction in stem height and stem girth experienced by seedlings subjected to waterlog treatments may be due to reduced photosynthetic activities since seedlings in this condition suffered water saturation and low oxygen level in the soil, thereby leading to reduced root permeability. It was also observed that the number of leaves in both Red Ixora and Yellow Ixora cultivars were not insignificant as it favoured the Yellow Ixora more than Red Ixora (Table 4). However, a varying number of leaves and branches in plants under stress (waterlog) was reported by Riaz et al. [31] when they reported that the reason for this variance may be due to flood inhibiting growth in association with changes in cell size and division resulting in reduced leaves production and promoting senescence within each cultivars and the promptness of one cultivar to solve this may be a key adjusting techniques of one cultivar ahead of the other. More so, the leaf area of Yellow Ixora is significant over the Red Ixora; while Red Ixora was significant in leaf area ratio over Yellow Ixora (Tables 7 and 8). Thus, reduction in leaf area and increase in leaf area ratio in Red Ixora, showing the occurrence of partial senescence; thus agreeing with the work of Al-Imran and Timothy [45], when they worked on Triticum aestivum (wheat) and they attributed the reduction in leaf area to facilitated senescence and abscission of leaves while Rahman [46], reported that it is due to reduction in stomata conductase leading to stomatal resistance. From the results, it was observed that fresh weight of plant (leaves, stems and roots) in Yellow Ixora WTP was highly significant as against those of the Yellow Ixora CTR; similar results was also observed in Red Ixora CTR and WTP respectively (Tables 3-5). However, it was observed that Yellow Ixora in either waterlogged treatments or control had more leaf weights than the Red Ixora in either control or waterlogged treatments. The fresh weights of stem and roots also favoured the Yellow Ixora in either waterlogged or control than the Red Ixora in either control or waterlogged treatments (Tables 4 and 5). This finding was against the findings of Medina et al. [47] and Liu et al. [9], when they reported a decrease in the whole plant biomass and root biomass of some flood tolerant plant species such as Erythrina speciosa and Distylium chinense. Mielke et al. [48] and Davanso et al. [49] also reported similar weight lost in Annona glabra and Tabebuia avellanedae respectively. They attributed it to slow metabolic activity under hypoxia causing reduction, as lack of oxygen blocks mitochondrial electron transport, oxidation and energy production. Effects of Waterlogging on the Chlorophyll contentChlorophyll content (colouration of leaves) is an important parameter to screen different cultivars for flood (waterlog) tolerance. Results regarding chlorophyll content shown in Table 19, indicated that there is no significant difference between Chloropyll-a, Chlorophyll-b and Total Chlorophyll of Control and Waterlog Treated Red Ixora Cultivar at end of the sixth month after treatment but in Yellow Ixora, there was a significant difference between Control and Waterlog Treated Plants. The lower chlorophyll content in Yellow Waterlog Treated Ixora may have compensated for early and better flowering and growth than Yellow Control and Red Ixora (CTR and WTP) plants. The results however revealed that at the first month of the experiment there seems to be no significant difference between these parameters. This was supported by Huang et al. [50] when they reported that Chlorophyll fluorescence kinetics react to the intrinsic characteristics of photosynthesis and can rapidly and sensitively reflects a plant’s physiological status and its relationship with the environment.Results of the present study (Table 9) also showed a contrast with findings in wheat crop under water stress conditions where water stress caused a significant reduction in net CO2 and photosynthesis [31,51-55] resulted in reduced biomass of plants. Under flood stress, there is always alteration in the normal stomatal opening and closing, thereby causing reduction in the leaf photosynthesis [56,57] was also contradicted in this study.
5. Conclusions
- From the findings of the study, it could be concluded that Ixora coccinea is a waterlogged tolerant plant because 100% survival rate was recorded. This was because the plant developed adventitious roots and hypertrophic lenticels which improved their oxygen exchange rate and thus improve their photosynthetic activity. The performances of Red Ixora and Yellow Ixora in either control or waterlogged treatments were similar. However, Yellow Ixora showed more adaptive efficiency than the Red Ixora. Thus, these survival responses (morphology and physiology) could be attributed to a combine effect of Ixora coccinea to waterlogging. Based on this, it could be recommended that Yellow Ixora should be considered first in the beautification and improvement of waterlog prone areas especially Lagos state, Nigeria. Hence, further research should be conducted on the effects of waterlogging on phytochemistry of I.coccinea.
ACKNOWLEDGEMENTS
- Authors extend gratitude to Mr. Otun, of the Department of Biochemistry, Lagos State University, Ojo, Lagos, Godonu Emmanuel, Ogun Gabriel and Setonji Benjamin for their supports during the field and bench works, and lastly to our anonymous reviewers for their editorial insights..
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML