-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 0(1): 11-16
doi:10.5923/j.plant.20201001.02

Hexanic Extracts of Trametes versicolor (L.:Fr.) Pilát to Control of Tomato Powdery Mildew
Ernesto Hernández Mendieta1, Marcelo Acosta Ramos2, Antonio Segura Miranda3, Dagoberto Guillén Sánchez4, Catalina Rubio Grandos5
1Investigador Estancia Posdoctoral, Programa de Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Km 38.5 Carr, México-Texcoco, Chapingo, Estado de México, México
2Profesor Investigador, Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo, Km 38.5 Carr, México-Texcoco, Chapingo, Estado de México, México
3Profesor Investigador, Programa de Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Km 38.5 Carr, México-Texcoco, Chapingo, Estado de México, México
4Profesor Investigador, Escuela de Estudios Superiores de Xalostoc, Universidad Autónoma del Estado de Morelos, Av. Nicolás Bravo s/n, Parque Industrial Cuautla, Xalostoc, Ciudad Ayala, Morelos, México
5Investigación y Desarrollo, Grand Mend Mexico Ltd, Netzahualcóyotl 214. Piso 3, Oficina 6-A. Col. Centro, Texcoco, Estado de México, México
Correspondence to: Ernesto Hernández Mendieta, Investigador Estancia Posdoctoral, Programa de Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Km 38.5 Carr, México-Texcoco, Chapingo, Estado de México, México.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The powdery mildew on tomato (Leveillula taurica) is one of the most important disease of this crop in Mexico, causing losses up to 40% on yield. There are no resistant tomato cultivars, so their control is based on use of chemical fungicides, mainly triazole fungicies. With the objective of obtaining new alternatives to control of this desease, concentrations of 5, 7.5 and 10% (v/v) of Hexanic extracts of Trametes versicolor developed on Luffa aegyptiaca were evaluated comparing their effectiveness (Abbott) with Amistar Gold® (0.5 L·Ha-1), Serenade Max® (5.0 kg·Ha-1) and a Control. Three applications were made every 7 days and the experimental design used was randomized complete blocks with 4 replicates. The percentage of damaged leaf area was evaluated with an Exprofeso scale with 0 to 6 indices 7 days after the first and second application and 7, 14 and 21 days after the third. The results obtained indicate that hexanic extracts offers a control of L. taurica, with average control of 47.41 and 57.82% and maximum of 58.16 and 65.09% on concentrations of 7.5 and 10% respectively, suggesting reduce the application interval from 7 to 5 days. The lowest concentration of extracts offered the less control of disease. L. aegyptiaca is considered to be an excellent substrate for the development and obtaining of mycelium of T. versicolor (R2: 0.9867). Phytotoxicity symptoms were not detected on the tomato crop cv Cid after applications of Hexanic extracts concentrations.
Keywords: Trametes versicolor, Hexanic extracts, Leveillula taurica, Luffa aegyptiaca
Cite this paper: Ernesto Hernández Mendieta, Marcelo Acosta Ramos, Antonio Segura Miranda, Dagoberto Guillén Sánchez, Catalina Rubio Grandos, Hexanic Extracts of Trametes versicolor (L.:Fr.) Pilát to Control of Tomato Powdery Mildew, International Journal of Plant Research, Vol. 0 No. 1, 2020, pp. 11-16. doi: 10.5923/j.plant.20201001.02.
Article Outline
1. Introduction
- Tomato production in Mexico grew at an average annual rate of 3.3% between 2005 and 2015, to reach 3.1 million tons. During that period, the total area allocated decreased at an average annual rate of 3.8%. In 1980, 85,500 ha were sown, in 2000, 75,900 ha and in 2015 the area was 50,596 ha [1].The decline in the planted area derives from the decrease in the area cultivated in the open sky, while cultivation under protected agricultural conditions continues to expand. The volume of red tomato obtained with the use of these latest technologies increased from 2.9% in 2005 to 32.2% in 2010, and reached 59.6% of the total volume in 2015 [1].Powdery mildew diseases are caused by fungus that infect leaves, stems, flowers and fruits in nearly 10,000 species of angiosperms [2]. Leveillula taurica was identified for the first time Mexico in the state of Sinaloa [3] and can now be detected throughout the country. L, taurica is an endoparasite that forms endophytic and epiphytic mycelia with conidiophores branches that grow through stomata [4]. The main symptoms are yellow lesions on the upper surface of the leaf with a dusty-looking sporulation that appears on the underside. In Canada, L. taurica infections caused considerable yield losses in greenhouse tomatoes [5], while the field has reported losses of up to 40%, as well as sunburns on fruits due to defoliation severe in the United States [6]. There are no cultivars resistant to this pathogen in the market [7], so the use of fungicides remains the main control method. In tomato, it can be controlled by the use of chemical fungicides, biological products or, by acquired systemic resistance (RSA) [8-12].At present, the use of fungicides such as propiconazole, penconazole, hexaconazole, triadimephon, traidimenol, tridemorf, dinocap and sulfur are the most efficient alternative for the control of powdery mildew [13], as well as new fungicides. generation as the case of the active ingredients belonging to the strobilurin group. The application of inorganic products such as Potassium Silicate (K2SiO3) and Monopotassium Phosphate reduce the severity of L. taurica in tomatoes by promoting resistance mechanisms [14-16]. The objective of this research was evaluate different concentrations of Hexanic extracts of Trametes versicolor as an alternative for the control of Leveillula taurica in tomato.
2. Materials and Methods
2.1. Research Location
- The production of mycelium and obtaining Hexanic extract of Trametes versicolor was carried out in the Pesticide Laboratory of the Postgraduate Program in Plant Protection of the Autonomous University of Chapingo in Chapingo, Mexico; while the field phase was carried out in the Municipality of Coatepec Harinas, State of Mexico, Mexico on tomato cv Cid on flowering stage in greenhouse conditions.
2.2. Mycelium Production of Tramtes versicolor
- The mycelium used to obtain the extract was obtained from the development of T. versicolor strain Mo008 on slices of vegetable sponge (Luffa aegyptiaca). 15 slices with average weight of 29.11 g were cut and sterilized under steam pressure at 15 lb by 20 min, subsequently dried at 50°C in an oven and placed in sterile polyurethane containers of 1 L capacity. Each slice was inoculated with 10 dics of 6 mm diameter of Malt-Agar Extract culture medium with T. versicolor of 20 days of development, which were randomly placed inside them. The inoculated slices were incubated for 90 days with 12 h of light / dark at 25°C in a BOD-20® incubator. The weight of the container was recorded and subsequently weighed at five-day intervals until the incubation period was completed. The percentage degradation of L. aegyptiaca by T. versicolor was estimated by equation 1.
2.3. Obtaining the Hexanic Extracts
- At 90 days of incubation, 150 g of L. aegyptiaca with development of T. versicolor were weighed and cut into small pieces and then macerated in a mortar by previously adding liquid nitrogen. The macerate was transferred to a 500 mL ball flask and 250 mL of Hexane was added. The flasks were placed on an oscillator for 48 h. Subsequently, the maceration was filtered and the Hexane was separated from the extract by means of a broken DLab® RE100-Pro steam at 50°C and 100 rpm. Once the solvent was removed, 150 mL of sterile double-distilled water was added and the flask was rinsed to subsequently transfer the extract to 200 mL amber bottles and store at room temperature in total darkness.
2.4. Treatments Evaluated
- Concentrations of 5, 7.5 and 10% (v / v) of the T. versicolor Hexanic extracts were evaluated, comparing their biological efficacy with the rate of 0.5 L·Ha-1 of Amistar Gold® SC (azoxystrobin + diphenoconazole [Syngenta®]) and 5.0 kg·Ha-1 of Serenade Max® (Bacillus subtillis strain QST713 [Bayer CropsCience®]) and a Control. An experimental design of randomized complete blocks with four repetitions was used, where each experimental unit consisted of a groove of 1.8 m wide by 5 m of long, equivalent to 9 m2 by repetition and 36 m2 by treatment. Three applications were made each seven-day with a pressurized CO2 equipment with a CD33 full cone nozzle (TeeJet®) with an expense of 633 L·Ha-1.
2.5. Evaluation of Biological Effectiveness
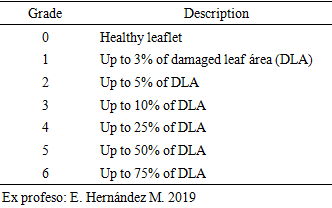
- An Exprofeso diagramatic scale was used to mesuring the level of infection of Leveillula taurica on leaflets on greenhouse-grown plants with levels from 0 to 6 (Table 1). The infection of L. taurica was recorder at 0 and 7 days after the first and second spray and at 7, 14 and 21 days after the third, randomly sampling 15 leaflets by experimental unit (60 per treatment) of the middle third of the plant. The infection (%) was determined using the Townsend and Heuberger formula (equation 2 [17]). The variance analysis and the Tukey means comparison test with a level of significance of 5% with the SAS® statistical analysis software were applied to the infection percentage data. The control efficacy of each treatment was determinated with equation 3 [18].
|
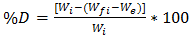 | (1) |
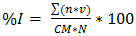 | (2) |
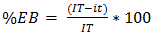 | (3) |
3. Results and Discussion
3.1. Degradation of Luffa aegyptiaca
- The degradation of the vegetable sponge began after 15 days of inoculation and to 90 days it was degraded by 71.75% on average. The degradation of L. aegyptiaca by T. versicolor its represented by a non-linear model (Figure 1) with R2 of 0.9867.
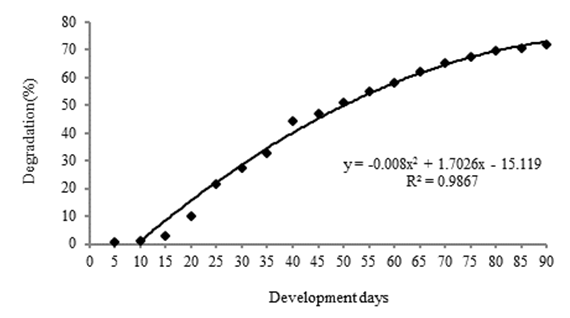 | Figure 1. Distribution of the degradation of Luffa aegyptiaca slices by Trametes versicolor |
3.2. Biological Efficacy Againts Leveillula taurica
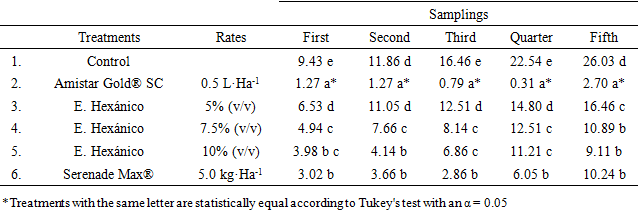
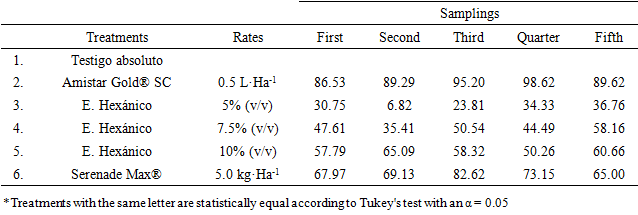
- The application program began with a mean infection of 1.98%, observing that of the evaluated treatments, the one that presented the lowest infection was the commercial dose of 0.5 L·Ha-1 of the mixture of azoxystrobin + diphenoconazole (Amistar Gold® SC) as well as the dose of 5.0 kg·Ha-1 of Bacillus subtilis (Serenade Max®), which presented an infection level of 0.79 and 2.86% respectively after three applications (Figure 2).The concentrations of the Hexanic extracts of T. versicolor showed a control effect of L. taurica however, as the infection increases in the Control, it also increases in each of the concentrations evaluated. The 10% concentration presented the lowest infection with a range of 3.98 to 9.11%, 21 days after the third application. The 7.5% concentration presented an infection statistically equal to that recorded in the 10% dose in the third, fourth and fifth evaluation and 21 days after third application, both concentrations were statistically equal to the treatment with B. subtilis (Table 2). The concentration of 5% (v / v) of the Hexanic extract presented the highest infection in all evaluations carried out.
|
|
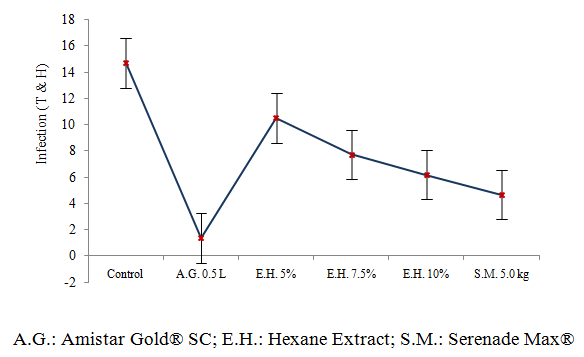 | Figure 2. Average distribution of Infection (T&H) in the treatments evaluated to control of tomato powdery mildew (Leveillula taurica) |
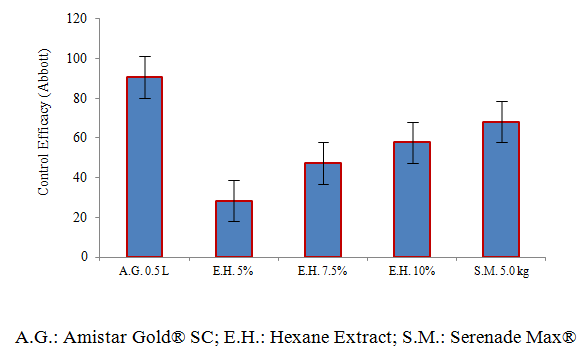 | Figure 3. Average distribution of Control Efficacy (Abbott) on treatments evaluated to control of tomato powdery mildew (Leveillula taurica) |
4. Conclusions
- The Hexanic extracts of Trametes versicolor presented a biological efficacy against the development of Leveillula taurica in tomato crop, detecting a direct relationship between the evaluated concentration and its control efficacy (Abbott), exceeding the concentrations of 7.5 and 10%, which they showed an average control of 47.41 and 57.82% respectively after three applications, suggesting to reduce the sprays interval from seven to five days. On the other hand, Luffa aegyptiaca is a substrate that favors the development of T. versicolor and can be used to the production of mycelium of this Basidiomycete. The tomato crop on which the Hexanic extracts of T. versicolor were sprayed at the concentrations evaluated showed no symptoms of phytotoxicity during the development of the investigation.
ACKNOWLEDGEMENTS
- To the National Council of Science and Technology (CONACYT) for the support granted to carry out the Postdoctoral Stay, as well as the Master's Program in Plant Protection of the Autonomous University of Chapingo, for the facilities granted to carry out the present investigation.
Dedication
- This research is dedicated to my daughters, Sofia Alejandra Hernández Díaz and Cristina Hernández Rubio with all my love (E. Hernández M.)
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML