-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2020; 10(1): 1-10
doi:10.5923/j.plant.20201001.01

Chemical Composition of Moringa oleifera Lam. and Moringa stenopetala Bac. Leaves from Kenya
Nathaniel Ebenezar Udofia 1, Onyancha Jared Misonge 2, Mugambi Mworia 1, Ncene William 1, Moriasi Gervason Apiri 2
1Kenya Methodist University, Meru
2Mount Kenya University, Thika
Correspondence to: Nathaniel Ebenezar Udofia , Kenya Methodist University, Meru.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The species of Moringa are native to India and Africa. However, they are cultivated in Tropical and sub-tropical countries mainly for nutrition and health products. In Kenya, most research has been done on M. oleifera and little on M. stenopetala. The paucity of consistent information of comprehensive chemical composition of the two species is generally observed. The purpose of this study was to find out the phytochemical and nutritive elements in M. oleifera and M. stenopetala collected from Kilifi and Isiolo counties in Kenya respectively. Standard chemical evaluation methods were used to establish qualitative and quantitative chemical composition of the two species in this study. Qualitative screening revealed alkaloids, cardiac glycosides, flavonoids, phenolics, phytosterols, saponins, tannins, coumarins, terpenoids, carbohydrates, proteins, lipids and vitamins. Proximate chemical composition revealed significant difference in composition of carbohydrates, flavonoids, phenols, alkaloids, saponins ash and crude fibre between M. oleifera and M. stenopetala leaf powder at p ≤ 0.05. However, there were no remarkable differences in the composition of crude lipids, crude proteins and moisture content in the leaf powders of M. oleifera and M. stenopetala. The study revealed essential and non-essential amino acids and no significant differences in composition at p ≤ 0.05 between the leaf powders of M. oleifera and M. stenopetala. Remarkable differences in composition of Thiamine (Vitamin B1) between M. oleifera and M. stenopetala were also noted (p < 0.05). However, there were no difference in composition vitamins (B6, B9, A and C) between the leaf powder of M. oleifera and M. stenopetala at (p < 0.05). Finally, the composition differences of calcium and potassium were significant between M. oleifera and M. stenopetala leaf powders at (p < 0.05), while there were no outstanding differences in the composition of copper, manganese, iron, zinc, magnesium and potassium between M. oleifera and M. stenopetala leaf powders.
Keywords: Medicinal plants, Moringaceae, Isiolo County, Kilifi County, Phytonutrients, Secondary metabolites
Cite this paper: Nathaniel Ebenezar Udofia , Onyancha Jared Misonge , Mugambi Mworia , Ncene William , Moriasi Gervason Apiri , Chemical Composition of Moringa oleifera Lam. and Moringa stenopetala Bac. Leaves from Kenya, International Journal of Plant Research, Vol. 10 No. 1, 2020, pp. 1-10. doi: 10.5923/j.plant.20201001.01.
Article Outline
1. Introduction
- Moringaceaea family constitute of three genus namely Anoma, Hyperanthrera and Moringa [1]. The Moringa genus has thirteen species of trees and shrubs [2]. All the species originated from India and Africa, though they have been cultivated in other tropical and sub-tropical countries. The species in this family have long been used by humanity for varied purposes including food, human and livestock medicine, water purification and cosmetic products. Of all the Moringa species, M. oleifera is the most researched and developed while the rest are considered ignored species [3]. M. oleifera is native to Himalayas but cultivated by many Kenyan farmers for nutrition and health. The Moringa species that are native to Kenya are M. stenopetala, M. arborea, M. borziana, M. rivae and M. longituba, of which M. stenopetala has attracted more studies due to its extensive use in traditional medicine [1]. The existing literature reports indicate varied composition of nutrients and functional elements of Moringa species from different study regions, while the scientific studies on nutritional and antinutritional composition of M. oleifera and M. stenopetala leaf powder samples collected from Kenya is scanty, the current study provides the chemical, nutritional and elemental composition of leaf powder of M. oleifera Lam. and M. stenopetala Bac collected from Kilifi and Isiolo Counties respectively.
2. Materials and Methods
2.1. Collection and Preparation of Plant Materials
- Fresh leaves of Moringa oleifera and Moringa stenopatala were collected from Isiolo county headquarters and Kilifi county headquarters respectively. The collected materials were authenticated with a taxonomist and the voucher specimens (MOEU2018 for M. oleifera and MSEU2018 for M. stenopetala) were deposited at the East African herbaria at the National museums of Kenya.
2.2. Phytochemical Investigation
2.2.1. Qualitative Phytochemical Screening
- Test for TanninsAbout 5 g of the powdered test plant materials were separately boiled with 20 ml of distilled water in test tubes and then filtered. 3 drops of 0.1% FeCl3 were added to the resulting filtrates. The appearance of bluish- green precipitate indicates presence of tannins [4].Test for Saponins by Frothing methodAbout 5 g of the powdered test plant materials was separately boiled with 20 of distilled water respectively in test tubes and then allowed to cool. After shaking, the appearance of frothing is indicative of the presence of saponins [4].Test for AlkaloidsApproximately 0.8 g of the crude plant powders were separately mixed with about 10 ml of 1% HCL, warmed and filtered. 2 ml of filtrates were treated with Mayer’s reagent. The appearance of cream-colored precipitate is a positive indication for the presence of alkaloids. Similarly, 2 ml of the filtered crude plant material were treated with Dragendorff’s reagent. A reddish-brown precipitate indicates a positive test for presence of alkaloids [4].Test for GlycosidesKeller-killiani test (test for deoxy sugars)The 1 g of powdered plant materials were extracted with 10 ml of chloroform and evaporated to dryness in test tubes. 0.4 ml of glacial acetic acid containing trace amour of ferric chloride was added followed by careful addition of 0.5 ml of concentrated sulphuric acid by the side of the test tube. Acetic acid layer showing a blue colour is a positive test for presence of cardiac glycosides [4]. Borntrager’s testThe test plant materials (0.5 g) were boiled separately with 1 ml of sulphuric acid in test tubes for 5 minutes. They were then filtered while hot, cooled and then shaken with equal volume of chloroform. The lower layers of chloroform were separated and shaken with half of their volumes with dilute ammonia. A rose pink to red colour produced in the ammoniacal layer is a positive indication of the presence of glycosides [4].Modified Borntrager’s testTwo drops of Kedde reagent were added to portions of dry crude plant materials. The presence of purple colour indicates the presence of glycosides whose aglycone moiety has unsaturated lactone ring [4]. Test for steroidsOne milliter solution of ethanol extract will be taken and then the Liebermann– Burchard reagent added. If reddish purple color is produced, it indicates the presence of steroids [4].Test for flavonoidsA few drops of concentrated hydrochloride acid will be added to a small amount of an alcoholic extract of each test plant material. Immediate development of a red color indicates the presence of flavonoids. The presence of flavonoids was also confirmed by three other methods. 10 ml solution of the extract was hydrolyzed with 10% sulfuric acid and divided into three portions: (1) 1 ml dilute ammonia solution was added in one portion. Greenish yellow colour indicates the presence of flavonoids; (2) 1 ml dilute sodium carbonate solution was added into one portion. Pale yellow indicates the presence of flavonoids; (3) 1 ml dilute sodium hydroxide solution was added into another portion. A yellow color indicates the presence of flavonoids [4].Test for PhenolsAbout 1 g of the leaf powder were boiled with 10 ml of 70% ethanol in a water bath using boiling tubes for 5 minutes. The extracts were filtered while hot and cooled. To 2 ml of these extracts, 5% Ferric chloride was added. The occurrence of a green precipitate indicates presence of phenols [4].Test for terpenoidsAbout 2 ml of the test extracts (alcoholic) were added to 1 ml of acetic acid anhydride followed by careful addition of concentrated sulphuric acid. The formation of a blue-green ring indicates the presence of terpenoids [4].
2.2.2. Quantitative Phytochemical Screening
- Determination of flavonoids To determine flavonoids, 5 g of each plant sample was weighed in a 250 ml titration flask, and 100 ml of the 80 % aqueous methanol was added at room temperature and shaken for 4 hours in an electric shaker. The entire solution was filtered through Whatman filter paper number 1. To the filtrate, 100 ml of the 80% aqueous methanol was again added at room temperature, shaken for 4 hours in an electric shaker then filtered as before. The filtrates were later transferred into weighed crucibles and evaporated to dryness over a water bath and weighed again [5]. The difference in weight gave the weights of flavonoids which were expressed as percentages of the weights of analyzed respective samples.
 Where W1 = Weight of empty crucible; W2 = Weight of crucible + flavonoids precipitate W0 = weight of sampleDetermination of alkaloids For alkaloids determination, 5 g of each sample was weighed into a 250 ml beaker, and 200 ml of 20% acetic acid in ethanol was added and allowed to stand for 4 hours. This was filtered and the extract was concentrated using a water bath to evaporate about one-quarter of the original volume. The concentrated ammonium solution was added dropwise to the extracts until precipitation was completed. Entire solutions were allowed to settle and washed using distilled water then filtered. The weights of respective precipitates were recorded and used to calculate the percentage of alkaloids in the tested samples [6].
Where W1 = Weight of empty crucible; W2 = Weight of crucible + flavonoids precipitate W0 = weight of sampleDetermination of alkaloids For alkaloids determination, 5 g of each sample was weighed into a 250 ml beaker, and 200 ml of 20% acetic acid in ethanol was added and allowed to stand for 4 hours. This was filtered and the extract was concentrated using a water bath to evaporate about one-quarter of the original volume. The concentrated ammonium solution was added dropwise to the extracts until precipitation was completed. Entire solutions were allowed to settle and washed using distilled water then filtered. The weights of respective precipitates were recorded and used to calculate the percentage of alkaloids in the tested samples [6]. Where W1 = Weight of empty crucible; W2 = Weight of crucible + Alkaloids precipitate W0 = weight of sampleDetermination of saponins Twenty grams (20 g) of each of the ground plant samples was weighed and added into 200 ml of 20% ethanol. The respective suspensions were heated over a hot water bath for 4 hours with continuous stirring at about 55°C. The mixtures were filtered and the residues re-extracted with another 200 ml of 20% ethanol. The combined extracts of each plant were reduced to 40 ml over water bath at about 90°C. The concentrates were transferred into 250 ml separating funnels and 20 ml of diethyl ether added followed by vigorous shaking. Aqueous layers were recovered while the ether layer was discarded. 60 ml of n-butanol was added to the aqueous layer. The solution of n-butanol extracts was washed twice with 10 ml 5% aqueous sodium chloride. The remaining solutions were evaporated on a hot water bath and dried in the hot air oven to a constant weight. The saponins content was calculated in percentage [6].
Where W1 = Weight of empty crucible; W2 = Weight of crucible + Alkaloids precipitate W0 = weight of sampleDetermination of saponins Twenty grams (20 g) of each of the ground plant samples was weighed and added into 200 ml of 20% ethanol. The respective suspensions were heated over a hot water bath for 4 hours with continuous stirring at about 55°C. The mixtures were filtered and the residues re-extracted with another 200 ml of 20% ethanol. The combined extracts of each plant were reduced to 40 ml over water bath at about 90°C. The concentrates were transferred into 250 ml separating funnels and 20 ml of diethyl ether added followed by vigorous shaking. Aqueous layers were recovered while the ether layer was discarded. 60 ml of n-butanol was added to the aqueous layer. The solution of n-butanol extracts was washed twice with 10 ml 5% aqueous sodium chloride. The remaining solutions were evaporated on a hot water bath and dried in the hot air oven to a constant weight. The saponins content was calculated in percentage [6].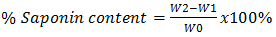 Where W1 = Weight of empty crucible; W2 = Weight of crucible + saponins precipitate W0 = weight of sampleDetermination of phenols To determine the total phenols, 5 g of each of the two plant samples was weighed into a 250 ml titration flask and 100 ml n-hexane added twice at 4 hours interval each. The filtrates were discarded for fat free sample preparation. 100 ml of diethyl ether was added to the residues, heated for 15 minutes, cooled up to room temperature and filtered into a separating funnel. 100 ml of the 10% NaOH solution was added and swirled to separate the aqueous layer from the organic layer. The aqueous layers were washed three times with 25 ml de-ionized water. The total aqueous layers were acidified up to pH 4.0 by adding 10% HCl solution and 50 ml dichloromethane (CH2Cl2) thus acidifying the aqueous layers in the separating flasks. Consequently, the organic layers were collected, dried and then weighed [7].
Where W1 = Weight of empty crucible; W2 = Weight of crucible + saponins precipitate W0 = weight of sampleDetermination of phenols To determine the total phenols, 5 g of each of the two plant samples was weighed into a 250 ml titration flask and 100 ml n-hexane added twice at 4 hours interval each. The filtrates were discarded for fat free sample preparation. 100 ml of diethyl ether was added to the residues, heated for 15 minutes, cooled up to room temperature and filtered into a separating funnel. 100 ml of the 10% NaOH solution was added and swirled to separate the aqueous layer from the organic layer. The aqueous layers were washed three times with 25 ml de-ionized water. The total aqueous layers were acidified up to pH 4.0 by adding 10% HCl solution and 50 ml dichloromethane (CH2Cl2) thus acidifying the aqueous layers in the separating flasks. Consequently, the organic layers were collected, dried and then weighed [7]. 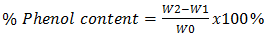 Where W1 = Weight of empty crucible; W2 = Weight of crucible + Phenols precipitate W0 = weight of sample.
Where W1 = Weight of empty crucible; W2 = Weight of crucible + Phenols precipitate W0 = weight of sample.2.3. Proximate Analysis
2.3.1. Determination of Moisture Content
- Two g of the powdered M. oleifera and M. stenopetala samples were placed in clean, dry respective crucibles and heated at 105°C in a hot air oven until a constant weight were attained. The moisture content of each sample was calculated as loss in weight of the original sample and expressed as percentage moisture content [8].
2.3.2. Determination of Crude Protein
- The crude protein was determined using the Kjeldahl method with slight modifications [9]. The following three steps were followed: Digestion: One g portions of each of the ground samples were weighed into separate digestion flasks. Reagent blank and high purity lysine hydrochloric acid were included to check of correctness of digestion parameters. 15 g potassium sulphate, 0.04 g anhydrous copper sulphate, and 0.8 g alundum granules were be added followed by 20 mL of concentrated sulphuric acid. The digestion flasks were placed on heating mantles and the mixture heated until white fumes cleared off the flask’s bulb. The mixtures were gently swirled, and heated further for 90 minutes in a fume chamber. The mixtures were allowed to cool to room temperature. Distillation: A mixture of 15 mL of hydrochloric acid and 70 mL of water (V/V HCl) was measured accurately to get acid standard solution then added to the titration flask. For reagent blank, 1 mL of acid and approximately 85 mL water were added followed by three drops of methyl red solution indicator. Slowly down side of flask, 100 ml of 45% sodium hydroxide solution was added to raise the PH of the mixture. The flask was connected to the distillation setup and distilled until at least 150 mL distillate was obtained in the titrating flask. Titration: Excess acid was titrated with standard 0.1M sodium hydroxide solution to orange endpoint (color changed from red to orange to yellow) and volume recorded to the nearest 0.01 mL (VNaOH). The reagent blank (B) was titrated in the same way. Calculations were performed using the following equation.
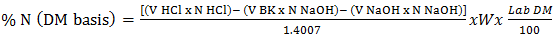 | (1) |
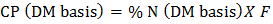 | (2) |
2.3.3. Determination of Crude Lipid
- The estimation was performed using the Soxhlet extraction method. 10 g of the powdered samples of M. oleifera and M. stenopetala were weighed and wrapped with a filter paper and placed in a thimble. The thimble was covered with cotton wool and placed in the extraction column that was connected to a Liebig condenser. 200 ml of n – hexane was used to extract the lipids from the study plants [8;9].
2.3.4. Determination of Crude Fibre
- Five grams of M. oleifera and M. stenopetala powdered materials were separately added to 200 ml of 1.25% H2SO4 and heated for 30 min in a hot water bath and filtered with a Buchner funnel. The respective residues were washed with distilled water thrice to free them of the acid. 200 ml of 1.25% NaOH was used to boil the residues for 30 minutes which were later filtered and washed three times with distilled water to make them alkaline free. The residues were then rinsed once with 10% HCl and twice with absolute ethanol. Finally, they were rinsed with petroleum ether three times to ensure fat free residues. The resultant respective residues were put in marked crucibles and dried at 105°C in a hot air oven overnight. After cooling in desiccators, the residues were ignited in a muffle furnace at 550°C for 90 minutes, cooled to room temperature and the weight of the ash obtained [8;9].
2.3.5. Determination of Ash Content
- Total ash content of a substance is the percentage of inorganic residue remaining after the organic matter has been burnt completely. 6 g of the pulverized plant samples were separately placed in labelled crucibles and ignited in a muffle furnace at 550°C for 6 hours. The resultant ashes were then cooled in a desiccator and weighed at room temperature to get the weight of the ash. The percentage of ash that was soluble in water, acid ad base was also determined [8;9].
2.3.6. Determination of Carbohydrate
- The carbohydrate content in the two plants tested was determined by subtracting the summed-up percentage compositions of moisture, protein, lipid, fibre, and ash contents from 100 [8].
2.3.7. Determination of Amino Acids in M. oleifera and M. stenopetala Leaf Powders
- Amino acid concentrations in M. oleifera and M. stenopetala leaf powders were determined according the methods described Okoronkwo et al. [9] with slight modifications. Briefly, five grams of M. oleifera and M. stenopetala leaf-powders were weighed and macerated in 50 ml in a water bath set at 50°C for 30 minutes. The mixtures were independently filtered through Whatman filter paper No. 1 and 5 ml aliquots taken and transferred into clean flasks. Then, 2.5 ml of 0.25% sodium carbonate and 5 ml of 1% Ninhydrin solution prepared in 95% ethanol were added and heated for 5 min in a hot water bath (95°C), cooled to room temperature and made up to the mark with distilled water. The standard amino acids were prepared in a similar manner as the test samples. The absorbance values were read by the help of a UV-Vis spectrophotometer, at wavelengths ranging from 204 nm to 350 nm with distilled water as the blank and used to determine amino acid concentrations in the assay samples using percent of 10 [9].
2.3.8. Determination of Vitamins in M. oleifera and M. stenopetala Leaf Powders
- Vitamin Aβ-carotene was analyzed following the procedure described by Bhatnagar-Panwar et al., 2013 with modifications [10]. Dry powder (10 mg) of M. oleifera and M. stenopetala were put in separate test tubes and 10 mL ethanol containing 0.1 g of butylated hydroxytoluene (BHT) was added to each. The test mixtures were then placed in a water bath at 70°C for 15 min and thereafter to each sample was removed from the water bath and saponified by adding 180 μL of 80% KOH. Each preparation in the test tube was then vortexed for 30 min to maximize extraction of carotene and their esters. The samples were placed directly on ice bath and 2.5 mL of de-ionized water and 2.5 mL Hexane/toluene mixture (10:8). Then the tubes were vortexed and then centrifuged at 2100 rpm for 5 min. The upper layer hexane/toluene fraction was then transferred to a separate test tube. The hexane/toluene extraction was repeated three times. The combined hexane/toluene fractions were dried using a Speed-vac concentrator. The residue was reconstituted in 200-400 μL Tetrahydrofuran (THF). The solution was filtered on a 0.2 μ nylon filter and 20 μL of the filtered solution was injected in the Shimadzu High performance liquid chromatograph. The mobile phase consisted of Acetonitrile: Methanol: Tetrahydrofuran (THF) (52:40:8) (v/v/v) at a flow rate of 2.0 mL min-1. The absorbance was recorded at 450 nm using Photodiode array detector (PDA). Peaks were identified by their retention time and absorption spectra were compared to those of known standards (β-carotene- Sigma Chemicals). β-carotene was quantified using peak areas of the authentic standard.Vitamin B groupThe Vitamin B groups were done following standard methods as described by AOAC 2004 [8].
2.3.9. Analysis of Elemental Composition
- The method was employed for the determination of mineral content. 2 g of the of the pulverized plant samples was placed in a crucible and ignited in a muffle furnace at 550°C for 6 hours. The resulting ashes were dissolved in 10 ml of 5% HNO3 and heated slowly for 20 minutes. After heating, they were filtered using Whatman filter paper No 1 and the filtrates used for the determination of mineral content. Atomic absorption spectrophotometer (AAS) was used to determine Ca, Cu, Mg, Fe, K, Zn, Mn, and Na levels in the samples. Respective standards were diluted according to their standard respective protocols. All assays were performed in three replicates for each species [8].
3. Results and Discussion
3.1. Qualitative Phytochemical Screening
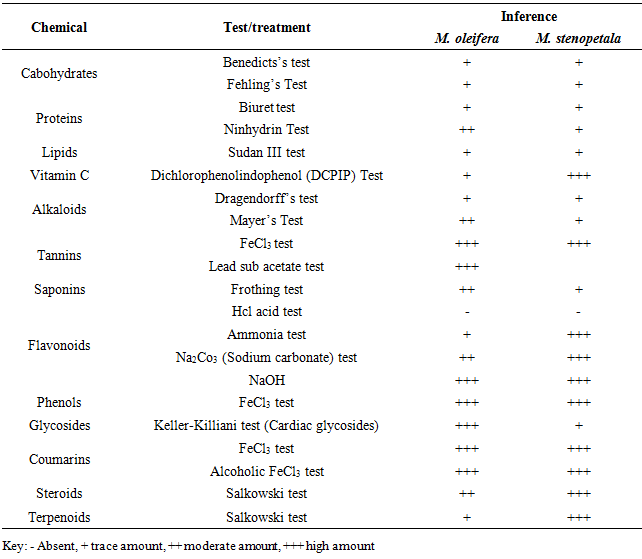
- Phytochemical screening revealed presence of pharmacologically active chemical compound groups in both M. oleifera and M. stenopetala. Alkaloids, cardiac glycosides, flavonoids, phenolics, phytosterols, saponins, tannins, coumarins and terpenoids. In addition, functional compound groups with nutrition value were also detected. These were carbohydrates, proteins, lipids and vitamins (Table 1). The compounds that were found in this study are in agreement with findings of other researchers from other parts of the world. For instance, reviews by [1;11]. Though, variation in the quantities of the detected phytochemicals were noted between M. oleifera and M. stenopetala, the differences are not far from the ones recorded [11;12 and 13].
|
3.2. Phytochemical Composition of M. oleifera and M. stenopetala Leaf Powders
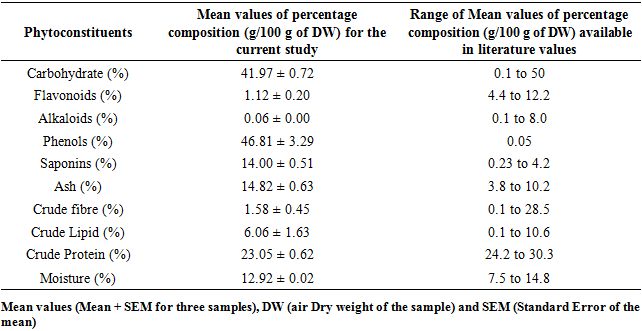
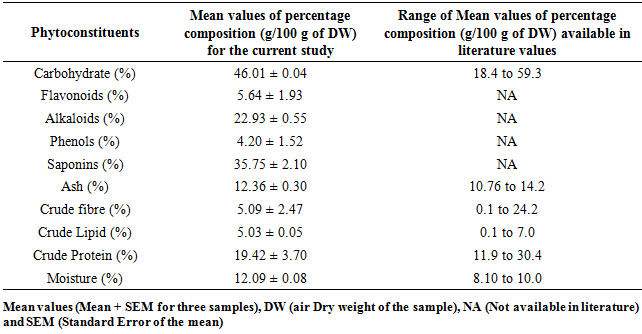
- Analysis of the composition showed different non-nutritive chemicals in both M. oleifera and M. stenopetala. The percentage composition of carbohydrates, saponins, crude fibre, crude lipids and moisture in M. oleifera concurred with earlier records Table 2 [14;15]. The values of flavonoid, alkaloid and crude protein were lower than those documented by other researchers while phenols and ash value were higher in M. oleifera Table 2. For M. stenopetala the studied phytochemical contents, carbohydrates, ash crude fibre, crude lipids, crude proteins and moisture were within the range values recorded by other researchers Table 3. However, flavonoid, alkaloid, phenol and saponin contents report from other works were not available in literature.
|
|
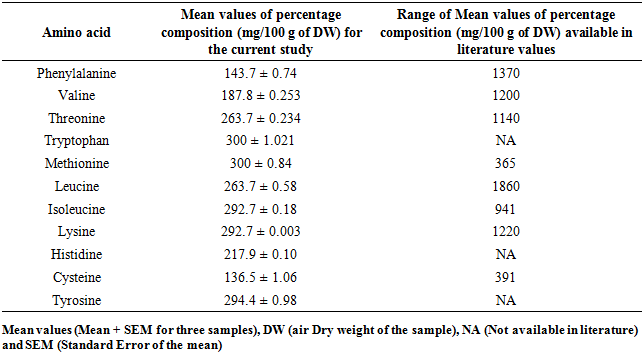
3.3. Composition of Selected Amino Acids in M. oleifera and M. stenopetala Leaf Powders
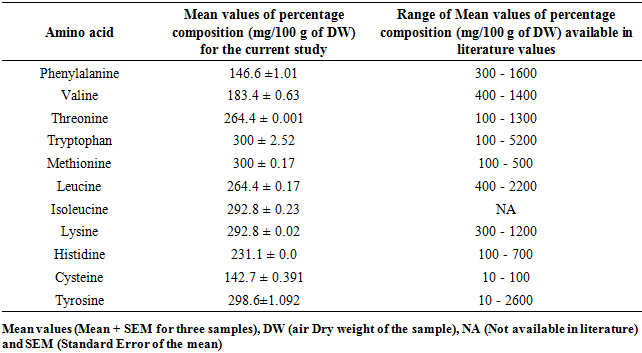
- This study disclosed different percentage composition of amino acids for M. oleifera and M. stenopetala leaf powders. For M. oleifera the levels of phenylalanine, valine, threonine, tryptophan, methionine, leucine, lysine, histidine, and tyrosine were within concurred with the values available in literature except for cysteine that were higher Table 4. Isoleucine was reported for the first time in this study for M. oleifera leaf powder.
|
|
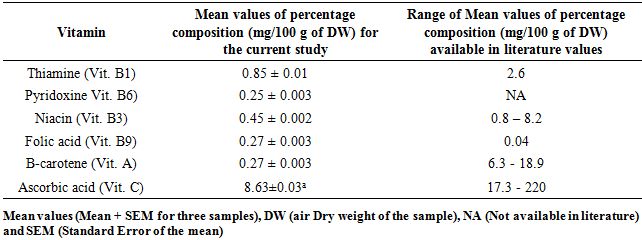
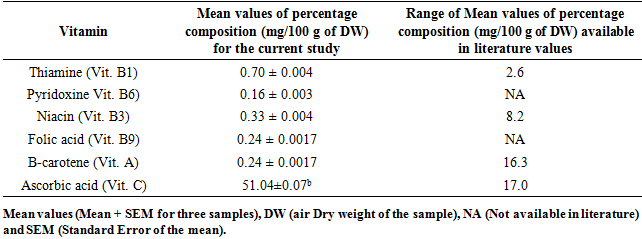
3.4. Composition of Some Vitamins in M. oleifera and M. stenopetala Leaf Powders
- Six different types of vitamins were assayed in this study. For M. oleifera, the values by composition of four of the vitamins studied were lower than the ones published in literature. Only Folic acid was found to have high concentration in this case Table 6 and 7. The composition of Thiamine (Vitamin B1) between M. oleifera and M. stenopetala revealed remarkable difference when they were compared statistically using t-test (p < 0.05). The composition of all other vitamins (B6, B9, A and C) were not different in percentage concentration between the leaf powder of M. oleifera and M. stenopetala at (p < 0.05) Table 6 and 7.
|
|
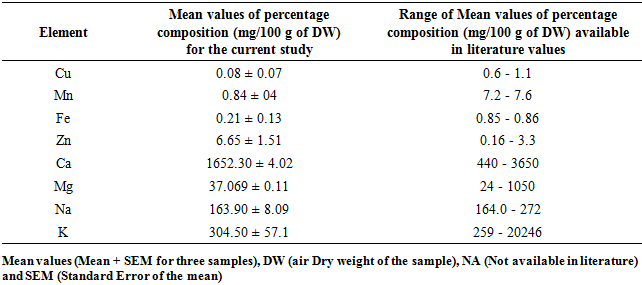
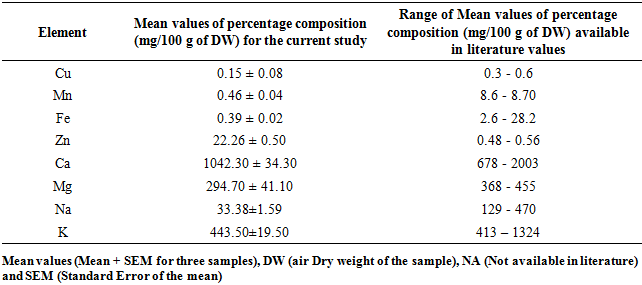
3.5. Composition Some Mineral Elements in M. oleifera and M. stenopetala Leaf Powders
- Moringa Oleifera and Moringa stenopetala in this study contain Copper (Cu), Manganese (Mn), Iron (Fe), Zinc (Zn), Calcium (Ca), Magnesium (Mg), Sodium (Na) and Potassium (K) at different concentrations as indicted in Table 8 and 9. The percentage composition of calcium and potassium were found to be within the range of published work for both M. oleifera and M. stenopetala, while that of magnesium and potassium composition for M. oleifera were in agreement with published reports. Concentrations of sodium, copper, manganese and Iron recorded in this study were lower while that of zinc were higher than the available literature reports for both M. oleifera and M. stenopetala Table 8 and 9.
|
|
4. Conclusions
- Based on the current study, it was concluded that phytochemicals, vitamins, amino acids and mineral elements support the use of leaf powders of M. oleifera and M. stenopetala for preventive and curative health care as well as food. The differences in chemical composition were attributed to environmental factors that influence accumulation of chemical and mineral elements in plants like climate, soil, altitude, age of development, parasites and time of harvesting.
ACKNOWLEDGEMENTS
- This research is part of PhD thesis work to be submitted in The School of Agriculture at Kenya Methodist University. The authors Mount Kenya University for providing laboratory facilities where this study was carried out. All individuals who provided plant materials from Isiolo and Kilifi County are acknowledged too. The technical assistance from Mr. Mandela Elias Nelson of the Department of Chemistry, Mount Kenya University are also appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML