-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2019; 9(2): 23-32
doi:10.5923/j.plant.20190902.02

Zanthoxylum Tsihanimposa: Used to Cure Measles in Madagascar and Analyzes of Phytochemicals, Antimicrobial Activities and Molecules in Leaves and Bark
Zafilaza Armand 1, Andriantsimahavandy Abel 1, Ramamonjisoa Daniel Joseph 1, Andrianainarivelo Mahandrimanana 2
1Department of Fundamental and Applied Biochemistry, Faculty of Sciences, University of Antananarivo, Madagascar
2Department of Inorganic Chemistry and Physics Chemistry, Faculty of Sciences, University of Antananarivo, Madagascar
Correspondence to: Zafilaza Armand , Department of Fundamental and Applied Biochemistry, Faculty of Sciences, University of Antananarivo, Madagascar.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

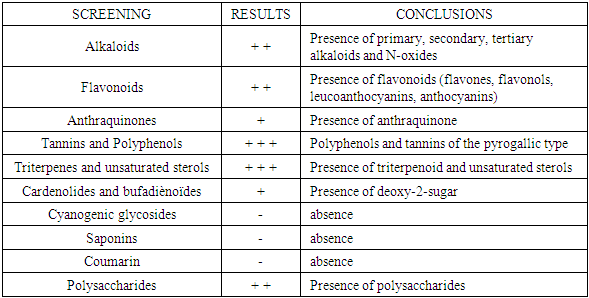
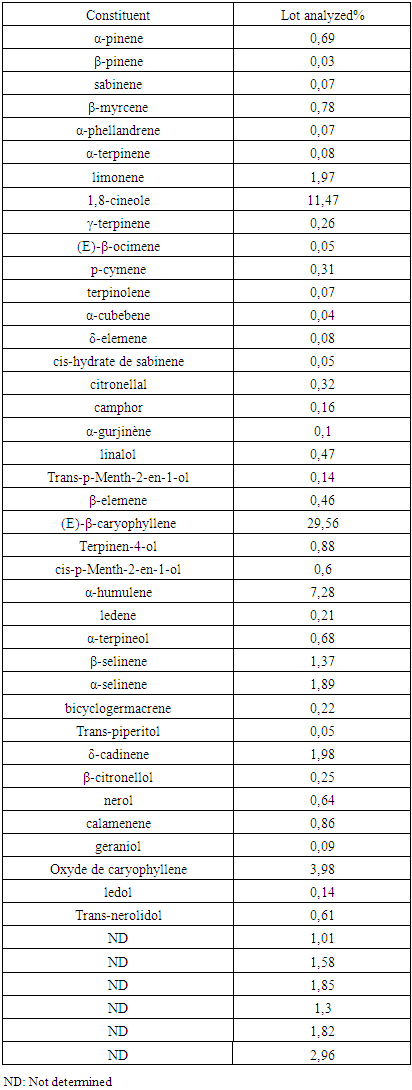
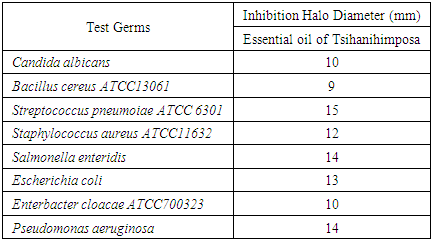
Zanthoxylum Tsihanimposa is a large tree 20 to 30 m tall, with a trunk of 60 cm. at 1 m and more in diameter. It grows in tropophilous forests, from 0 to 400 m. altitude, on calcareous or siliceous lands of the western slope. It is found in the West (Sector North) on the limestone hills and plateaus of Ankarano in the Ambongo-Boina drive: medium basin of Bemarivo (Boina), Firingalava between Maevatanana and Andriba (Boina) and Morataitra near Maevatanana, Tampoketsa of the Âmbongo (Kelifely plateau). In the basin of the Mahavavy of the West. In the Menabe sector on the infra-Cretaceous sandstones of the lower Tsiribihina basin and in the Sambirano region on Ankaramahifitry and Andampy mountain. The genus includes about 250 species in all warm regions, represented in the Malagasy region by two Mascarene species and six endemic species from Madagascar. The two species of Mascarenes, unlike those of Madagascar which are i-carpellées, normally have flowers with 4 carpels. There is the presence of primary, secondary, tertiary alkaloids and N-oxides. Flavonoids are in the form of flavones, flavonols, leucoanthocyanins, anthocyanins. And also polyphenols and tannins manifest as pyrogallic type. Zanthoxylum Tsihanimposa contains triterpenoids, unsaturated sterols and cradenolides, bufadienoides in the form of deoxy-2-sugar. But cyanogenic glycosides, saponosides, coumarins are absent compared to Polyssacharides. According to GPC analysis, the "Tsihanimposa" contains several interesting molecules. It is characterized by molecules (E) -β-caryophyllene 29.6%, 1,8-cineol 11.5% and α-humulene 7.3% which occupy nearly 50% of the constituents. The three elements are bicyclic natural sesquiterpenes composed of a 9-carbon macrocycle fused to a cyclobutane ring. And other molecules such as limonene 2.0%, caryophyllene oxide 4.0%, δ-cardinene 2.0% and α-selinene 1.9% occupy the other half of the constituents. The essential oil of Tsihanimposa has an effect on the bacteria tested. It is sensitive to Candida albicans with x (inhibition halo) is equal to 10mm; similarly for Bacillus cereus x is equal to 9mm. It is very susceptible to Streptococcus pneumoiae with x is equal to 15mm. Regarding Staphylococcus aureus, Salmonella enteridis, Escherichia coli, Enterbacter cloacae, Pseudomonas aeruginosa, they are susceptible to Tsihanimposa with inhibition halo x is equal to 12mm; 14mm; 13mm; 10mm; 14mm.
Keywords: Zanthoxylum Tsihanimposa, Rougeole, Phytochemical analysis, Anti-microbial activities, Molecular analyzes
Cite this paper: Zafilaza Armand , Andriantsimahavandy Abel , Ramamonjisoa Daniel Joseph , Andrianainarivelo Mahandrimanana , Zanthoxylum Tsihanimposa: Used to Cure Measles in Madagascar and Analyzes of Phytochemicals, Antimicrobial Activities and Molecules in Leaves and Bark, International Journal of Plant Research, Vol. 9 No. 2, 2019, pp. 23-32. doi: 10.5923/j.plant.20190902.02.
Article Outline
1. Introduction
- Zanthoxylum Tsihanimposa is a large tree of 20 to 30 m. high, trunk 60 cm. at 1 m. and more in diameter, often bearing on the base a few large (2 x 1.6 cm.) very thick spines and thick plates of stratified yellow cork, covering a whitish bark, thick, hot-smelling and with a strong balsamic odor, the flowering twigs and leaves unarmed at this stage; at the young trunk stage and branches covered with conical spines and leaves (rachis and median side of the lower page of leaflets) armed with thinner sharp spines; twigs with thick ends (10-12 mm in diameter); narrow leaf scars. Leaves grouped by 5-20 in bouquet at the top of the branches, developing after flowering, very large (55 to 80 cm.), With 9-16 pairs of opposite or subopposed leaflets, glabrous except traces of puberulenceon the rachis of very young leaves; subcylindrical rachis, plan above; petiolules very short or subnose, with a tightly compressed base; lower leaflets ovate (5-10 x 2.4-5.2 cm.), the next larger oblong or ovate-lanceolate (12-20 x 3 cm-1 cm), all more or less unequal to the base, rounded or obtuse, then cuspidate or acuminate at the summit; edges darkly and very finely wavy-crenellated; secondary veins and network visible on both sides; punctuations visible by direct light on the lower page of the leaflets; by transparency, very numerous points, more or less visible according to the state (young or adult) of the leaf, reddish and of 2 sorts, some smaller, scattered between the other bigger and more rare [1,11].Having thyrsoidal inflorescences, 8-1 5 cm long, grouped by 7-20 in terminal cluster, around the leaf bud which will develop a little later, 2-3 times branched, dense, entirely pubescent or puberulent; axis often carrying some sharp spines; bracts rounded, appressed, little distinct; short pedicels (o, 5-2 mm.), pubescent; flowers 4-mothers, rarely 5-mothers; ovate-obtuse sepals (1 x 0.7 mm.), equal, pubescent-ciliate, nested-decussed; oval-subacute petals (2.5 x i, 3 mm.), pubescent on the outer side, valvars-unduplicated; proceeding stamens as long as the petals; small anthers (1 x 0.7 mm); disc wide, erect collar, irregularly denticulate; rudiment of gynoecae lageniform, 1 mm high, attenuated truncated style. Inflorescences 9 less branched, sometimes shorter, with 4-mer, sessile or subsessile flowers; sepals and similar petals; no staminodes; bumpy disc, adorned at the base of the ovary: unicelled gynoecium, globular, glabrous, slightly contracted at the base; almost lateral style, short (0.5 mm.), with a truncated stigma, a little wider than the style. Its fruit is sub globular (8 mm), monosperm; exocarp stuffed with glands; cartilaginous endocarp detaching from exocarp to dehiscence; with a brilliant black seed; testa very hard; reddish integument; albumen white; right embryo. It flowers in the dry season, from September to November and gives fruit in December [1,11].Zanthoxylum Tsihanimposa grows in tropophilous forests, from 0 to 400 m. altitude, on calcareous or siliceous lands of the western slope. It is found in the West (Sector North) on the limestone hills and plateaus of Ankarano in the Ambongo-Boina drive: medium basin of Bemarivo (Boina), Firingalava between Maevatanana and Andriba (Boina) and Morataitra near Maevatanana, Tampoketsa of the Âmbongo (Kelifely plateau). In the basin of the Mahavavy of the West. In the Menabe sector on the infra-Cretaceous sandstones of the lower Tsiribihina basin and in the Sambirano region on Ankaramahifitry and Andampy mountain.The genus includes about 250 species in all warm regions, represented in the Malagasy region by two Mascarene species and six endemic species from Madagascar. The two species of Mascarenes, unlike those of Madagascar which are i-carpellées, normally have flowers with 4 carpels.Kingdom: PlantaeUnder-reign: TracheobiontaDivision: MagnoliophytaClass: MagnoliopsidaSubclass: RosidaeOrder: SapindalesFamily: RutaceaeGenus: ZanthoxylumSpecies: Zanthoxylum TsihanimposaVernacular name: Fahavalonkazo, Manongo, Manago [1,11].Zanthoxylum Tsihanimposa, Tsihanimosa, Manongo is used in the native pharmacopoeia. If the traditherapeuthes use it to treat malaria, fatigue and muscular pains by decoction of leaves and bark, Sakalava, they use it as an antibiotic to treat different diseases. Unfortunately, research on Zanthoxylum Tsihanimposa is rare.On the other hand, wood, often marketed under the name of "fahavalonkazo", is used locally in the construction of houses, especially for window frames and doors, as well as for shipbuilding and caisserie. It is also suitable for veneers and plywood [1,11].The heartwood is yellowish brown, sometimes with greenish or golden reflections, and isdistinctly distinct from the greyish white sapwood. The thread is straight; the grain is coarse.It is moderately heavy wood, with a density of 520-680 kg / m³ at 12% humidity. It dries quickly with little alteration. The shrinkage rates are moderately high, from green to oven dry 4.3-5.5% radial and 7.9-8.9% tangential. Once dry, it is moderately stable in use. At 12% humidity, the modulus of rupture is 120-161 N / mm², the modulus of elasticity 12 100-16 800 N / mm², axial compression 46-59 N / mm², shear 5 N / mm², splitting of 12-13 N / mm and Chalais-Meudon flank hardness of 1.9-3.6 [1,11]. Wood is relatively easy to saw and work. He holds nails moderately well, with satisfactory bonding and painting characteristics. Durability is low to moderate, with wood being prone to termite, Lyctus and fungus attacks. The heartwood is moderately resistant to impregnation with preservatives [1,11].Zanthoxylum Tsihanihimposa, Tsihanimosa, Manongo is used in the native pharmacopoeia. Traditherapeuthes use to treat malaria, fatigue and muscle aches. They practice decoction of leaves and bark. Sakalava use as an antibiotic to treat different diseases. But in 2018, measles manifests itself in the world, especially in Madagascar, where measles is widespread and kills almost 200 people. In front of the plague, the population of Sakalava Bezava resort to ancestral knowledge that is to say uses traditional medicine. They use the plant "Tsihanimposa" to cure measles. Research on Zanthoxylum Tsihanimposa is rare.In the past, measles reached hundreds of thousands of children every year throughout the world. Since the 1960s, widespread vaccination has allowed the virus to almost disappear in most developed countries.OMS is supporting efforts by Madagascar's Ministry of Public Health to respond to an unusually large measles outbreak. Madagascar had not experienced a measles outbreak since the 2003 and 2004 outbreaks, during which 62,233 and 35,558 cases were reported, respectively. The number of cases then declined sharply until the current outbreak. Between 4 October 2018 and 7 January 2019, the Ministry of Health of Madagascar notified 19,539 cases of measles, including 39 deaths in health facilities (case fatality rate: 0.2%). Cases were reported in 66 of the 114 districts in the 22 regions of the country. The outbreak spread to densely populated urban areas, including the cities of Toamasina, Mahajanga, Antsirabe, Toliara and Antananarivo, the capital. Children aged 1 to 14 account for 64% of all cases in this epidemic. Within this group, the age distribution is as follows: 35% for children under five, 22% for the 5-9 age group and 19% for the 10-14 age group. Both sexes are affectedto the same extent, the ratio of male to female cases was 1.04 [5]. The circulating viruses in this outbreak of measles in Madagascar are genotype B3, the genotype generally observed in Africa and Europe. However, neighboring countries have not reported any cases of measles in people who have traveled to Madagascar, and preliminary surveys in Madagascar have found no link to cases from countries affected by measles outbreaks in the Region. African or European [5].This outbreak of measles occurred at the same time as the seasonal resurgence of plague in the country, undermining public health interventions. It is caused by the measles virus, which belongs to the genus Morbillivirus, of the family Paramyxoviridae. Mononegavirales order with group II enveloped RNA virus with the name Measles virus. The virus is transmitted by direct contact and through the air, infecting the mucous membranes and spreading throughout the body. Measles is a strictly human disease with no animal reservoir [6-9].
2. Materials and Methods
2.1. Method of Work for the Ethnobotanical Study
- The working method consists of conducting ethnobotanical surveys. They did in three stages during the month of January 2019:- direct interview with the population at the age of 26 to 80 years.- interview with the 10 renowned traditional healers including six (6) men and four (4) women.- and the survey consists of the recommended recipes for the treatment of measles.
2.2. Ethno Medical Information
- Ethnomedical information was collected from traditional healers commonly referred to as "healers" in the study area. This species was identified and confirmed at the herbarium at the identification center in Tsimbazaza. The vernacular names are Fahavalonkazo, Tsihanimposa. Its leaves and bark are widely used in traditional medical use for the treatment of malaria, asthma and diabetes [1,12,13].
2.3. Phytochemical Screening
- The plant material consists of essential oils extracted from the leaves of Tsianimposa. The leaves were harvested in January 2018 in the dense forest of Andampy fokontany Ankotika. These organs were washed with running water and dried in an oven at 50°C for 5 days. The leaves were placed in the rotavapor for distillations and the extraction of essential oils. The detection tests for large groups of chemical compounds focused on residues. We used the analytical techniques described in the practice papers [14,15,27,28].a) Detection of sterols and polyterpenesSterols and polyterpenes were found in residues (R1) by the reaction of liebermann. An aliquot of residue is dissolved in heat in 1 ml of acetic anhydride in a capsule, then taken up in a test tube in which are poured 0.5 ml of concentrated H2SO4. The appearance of a violet color that turns blue then green indicates a positive reaction [14,15,27,28].b) Detection of tanninsTannoids (hydrolysable tannins) and true tannins (non-hydrolysable or condensed tannins) are polymers of polyphenols. These were evidenced by the iron (III) chloride reaction in the crude extracts (S1-S5).A few drops of an aqueous 2% FeCl 3 solution are added in 2 ml of crude extract. The appearance of a blue-black or green-black color respectively indicates the presence of tannoids or true tannins.True tannins were found in residues (R3-R5) by Stiasny's reagent. To an aliquot of the residue taken up in MeOH and then evaporated, are added 15 ml of the Stiasny reagent (30% CH2O in concentrated HCl 2/1). Then, the formation of flake precipitate after cooling, indicates a positive reaction.The true tannins were also heat-highlighted in the presence of concentrated HCl to an aliquot of residue taken up in 2 ml of distilled water, a few drops of concentrated CH 2 are added, the whole is heated in a boiling water bath. The formation of a red precipitate indicates a positive test.The detection of the tannoids was carried out in the residues (R3-R5): to the filtrates of the reaction masses saturated with CH3COONa are added a few drops of an aqueous solution of FeCl3 at 2%. The reaction is positive if a blue-black tint appears [14,15,27,28].c) Detection of flavonoidsFlavonoids were detected in residues (R1-R5) by the cyanidine reaction. To an aliquot of residue dissolved in 5 ml of hydrochloric ethanol (2: 1, v / v) are added two to three Mg chips (or 30-50 mg of Zn powder) and some drops of isopetanol. The appearance of an intense pink-orange or purplish coloration (red or red-orange with Zn) indicates a positive reaction [11,14,15,27,28].d) Detection of quinonesThe quinones were shown in residues (R1 - R5) by the Borntraëger reagent. An aliquot of residue dissolved in 5 ml of HCl diluted 1/5, is heated in a boiling water bath for 30 min, then extracted with 20 ml of CHCl 3 after cooling. To the organic phase are added 0.5 ml of 50% diluted NH4OH. The appearance of a hue ranging from red to purple indicates a positive reaction.e) Detection of saponinsThe saponins were detected in the residues (R1 - R5) by the foam test, then their presence is confirmed by the blood test and by the determination of the optical density (OD). The residues are taken up in 5 ml of distilled water and then introduced into a test tube. The test tube is shaken vigorously. The formation of a foam (height greater than 1 cm) stable, persisting for 1 hour indicates the abundant presence of saponins. The blood test was performed on the aqueous extracts of R4 and R5. Then, in a test tube containing 2 ml of fresh animal blood dissolved in physiological solution (aqueous solution of 0.9% NaCl), a few drops of aqueous extract are added. The observation of discoloration with respect to a control tube indicates a positive test. Then, in 3 test tubes, 1 ml of blood solution (1 ml of blood in 25 ml of isotonic solution) is introduced. One of the tubes serves as a witness. In each of the two remaining, are added respectively 5 and 10 drops of extracts to be tested. After homogenization, the contents of the test tubes are centrifuged for 10 min at 2000 g, then the OD of each sampled supernatant is measured using a colorimeter (wavelength 420 nm) [14,15,27,28].f) Detection of reducing sugarsThe reducing sugars were found in the crude extracts (S1 - S5) by Fehling's reagent and then confirmed by the Tollens test. To carry out the Fehling test, to 5 ml of crude extract are added 5 ml of Fehling liquor. The formation of a brick red precipitate after 2-3 minutes of heating in a water bath at 70°C indicates a positive reaction. The detection of reducing sugars by the Tollens test consisted in adding 5 ml of the Tollens reagent to 5 ml of crude extract. The formation of a silver mirror after a few minutes indicates a positive reaction [16].g) Detection of coumarinesCoumarins were detected in residues (R1 - R5) by reaction on the lactone ring. In 2 test tubes, 2 ml of ethanolic solution obtained from each residue are introduced. In one of the test tubes are added 0.5 ml of 10% NaOH, and the test tubes are heated in a water bath to boiling. After cooling, 4 ml of distilled water are added to each test tube. If the test tube liquid in which the alkaline solution has been added is transparent or more transparent to the liquid of the control test tube (without alkaline solution), then the reaction is positive. By acidifying the clear solution with a few drops of concentrated HCl, it loses its yellow color, becomes cloudy or a precipitate forms [11,14,15,27,28].h) Protein detectionProteins were detected in residues (R1 - R5) by the reaction of biuret. To an aliquot of the residue dissolved in 2 ml of 20% aqueous NaOH in a test tube are added 2 -3 drops of an aqueous solution of 2% CuSO4. The appearance of a violet color, sometimes with a reddish hue, indicates a positive reaction [11,14,15,27,28].i) Detection of alkaloidsThe alkaloids were detected in residues (R1 - R5) with Dragendorff and Burchard reagents (precipitation reagents): 0.1 g of residue is taken up in 6 ml of 60% ethanol and then distributed in 2 test tubes. In the first tube, 2 drops of Dragendorff reagent are added. The appearance of an orange-red or reddish-red precipitate indicates a positive test. In the second tube, 2 drops of Burchard's reagent are added. The appearance of a brown precipitate indicates a positive test [11,14,15,27,28].
2.4. Analysis of the Molecules
- Separation of molecules from a mixture by gas chromatography (GC) The sample (a volatile liquid) is first introduced at the top of the column via a micro-syringe that will pass through a soft pellet, called a septum, to end up in a small chamber upstream of the column called an injector. The injector is crossed by the carrier gas and brought to a temperature appropriate to the volatility of the sample. The quantities injected are in 51/50 ° split modes with integration of 0.02% air percentage threshold. Then, once made volatile, the various compounds of the sample will be washed away by the carrier gas through the column, in experiment; it is hydrogen gas is used with constant pressure at 0.50 bar and separate from each other depending on their affinity with the stationary phase. The stationary phase is a non (or slightly) volatile liquid (gas-liquid chromatography). It will cause a phenomenon of chromatographic retention with the different compounds called solutes. More the compound has affinitywith the stationary phase it will take longer to get out of the column. The gross experimental size is called the retention time, which is the time that elapses between the injection of the sample and the appearance of the maximum signal from the solute to the detector. To promote the transport of all compounds through the column (elution), the correct furnace temperature must be determined. In the experiment the temperature of the oven is 50°C to 250°C or 5°C / min. The temperature must be slightly higher than the boiling temperature of the compounds so that the compounds do not come out too early, which would have the consequence of having their peaks coincided with that of the dead time. The work must be in isotherm, ie with a fixed temperature during the whole analysis or with a temperature program which varies [10,29].At the exit of the column, the compounds meet an essential element which is called detector, the column used is UB-WAX (30m x 0.32mm x 0.5μm). This element continuously evaluates the amount of each of the separated constituents within the carrier gas by measuring different physical properties of the gas mixture. The detector sends an electronic signal to a recorder (sort of printer) which will draw the curves of each peak according to their intensity (Gaussian type curve) is the set of peaks is called chromatogram. In the experiment, the detector used is FID. Currently, software is increasingly replacing paper recorders for the interpretation of signals sent by detectors [10,29].
2.5. Antimicrobial Activity
- In the test of antimicrobial activity with Tsihanimposa many germs are used. These are Gram - and Gram + as well as pathogenic unicellular fungi. They came from the Laboratory of Microbiology of the Environment (LME) of the National Center for Research on the Environment (CNRE).Here is the list of germs used during the series of tests with their characteristics [2,20].• Gram- bacteria:Escherichia coli: called "colibacillus" this bacterium is the normal host of human intestines and warm-blooded animals. It is a faecal coliform, indicator of fecal contamination in water and food. It is sought after in butcher's meats, fishery products, raw and fermented milks.The strains responsible for infections in humans are different from those present in the intestinal flora. They are notably involved in diarrheal syndromes in adults and young children in developing and developed countries.They are classified as group 2 biological pathogens, that is, they can cause disease in humans and pose a danger to workers, but their spread to communities is unlikely. In addition, prophylaxis and effective treatment exist [17-20].Salmonella enteridis: it is an intestinal parasitic bacterium of the vertebrae. Its pathogenic strain is responsible for gastroenteritis during individual or collective food poisoning. These conditions, known as salmonellosis, are manifested by diarrhea, vomiting, and fever that develop 8 to 10 hours after ingestion of a contaminated food. This bacterium is also classified as a pathogen 2 [17-20].• Gram + bacteria:Bacillus cereus: living in the soil and in water, this bacterium can survive in the environment as spores. It can contaminate plant foods and many cooked dishes. It is one of the microbes coveted in raw peeled and cut plant products, ready-to-use, sprouted seeds, vegetable juice and raw fruit [17-20].Staphylococcus aureus: Staphylococci are spherical cocci isolated in clusters or diplococci. They are immobile, facultatively anaerobic in general and grow in nutrient agar or trypcase soy agar at 37°C and pH ranging from 7.2 to 7.4. They are of human or animal origin (poultry, cattle ...) and are pathogenic in group 2. The pathogenicity is:- by virulence: staphylococci produce surface proteins and enzymes including free coagulase and thermonuclease, thus causing a wide variety of infections including boils, pneumonia, ....- by toxinogenesis: Staphylococci produce various toxins including enterotoxins causing individual or collective toxi-infection due to contaminated food. This food poisoning is expressed in 2 to 4 hours by nausea, abdominal pain, repeated vomiting and diarrhea lasting 24 to 48 hours [17-20].Enterobacter cloacae: Enterococci are oval cocci, isolated or in diplococci, in chains or chains, both facultative and anaerobic. They are cultured at 37°C. and at pH 7.2 on commonly used media.These are ubiquitous germs found in soil, freshwater, marine and in living plants as commensals in the human gut and animals. They can be pathogenic for humans and animals by group 2. They are responsible for various opportunistic and dangerous human opportunistic infections such as endocarditis, sepsis, meningitis and urinary infections [17-20].• Pathogenic yeast fungiCandida albicans: colonies of this yeast are white to creamy beige convex on Sabouraud agar. It produces blastospores and chamydospores. It is sought during microbiological control of cosmetic products. Pathogen for humans (Group 2) and animals or an agent of mycosis of the skin or mucous membranes [17-20].
3. Results of Analysis
3.1. Ethnobotanical Study
- The interview we had with traditional healers made it possible to notice that the plant species fahavalonkazo or tsihanihimposa is used to cure various diseases. Sakalava uses a lot of herbs to treat thyroid fever, malaria, diarrhea, asthma and diabetes. The people of the commune of Antranokarany repaties 11 Fokontany know and use Fahavalonkazo or Tsihanimoposa a lot.
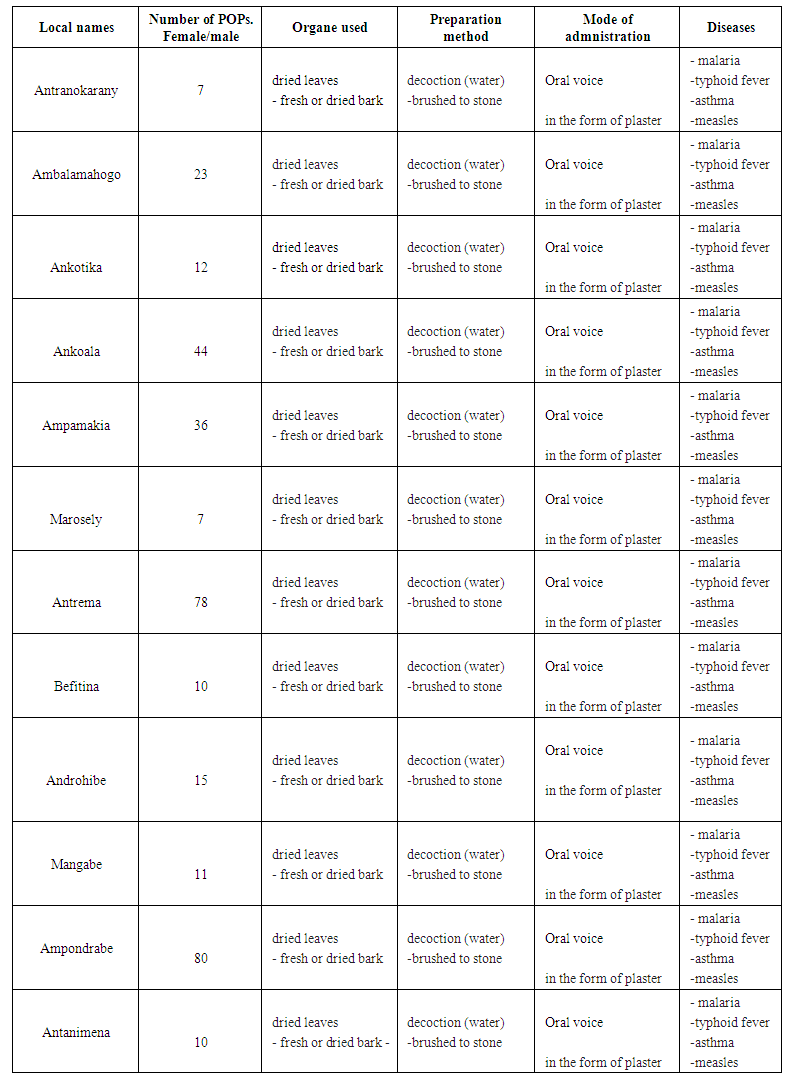 | Table 1. Representation of the ethnobotanical survey in the commune of Antranokarany |
3.2. Summary of Screens
3.3. Result of Separation of the Molecules of a Mixture by Gas Chromatography (GCP)
|
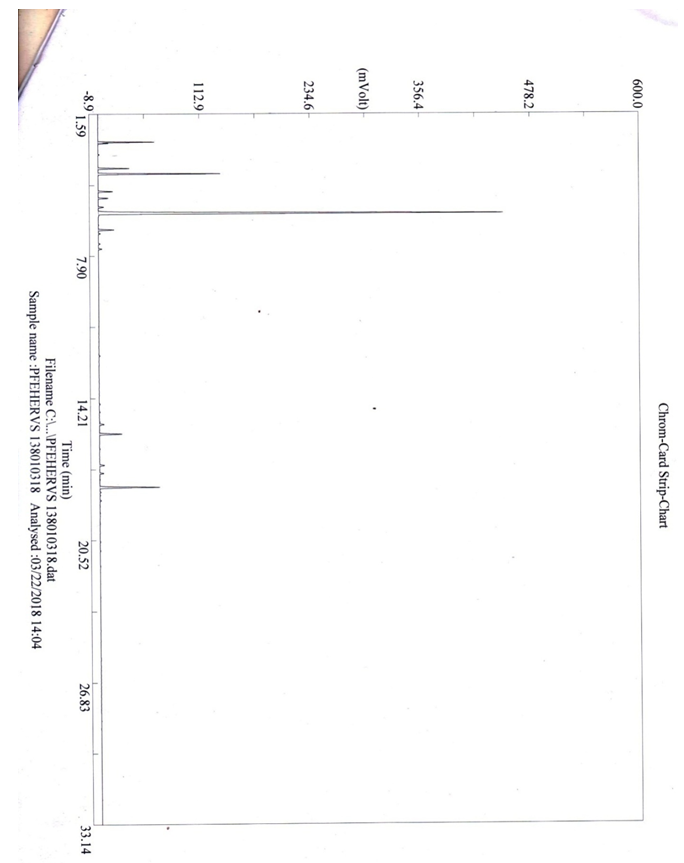 | Figure 1 |
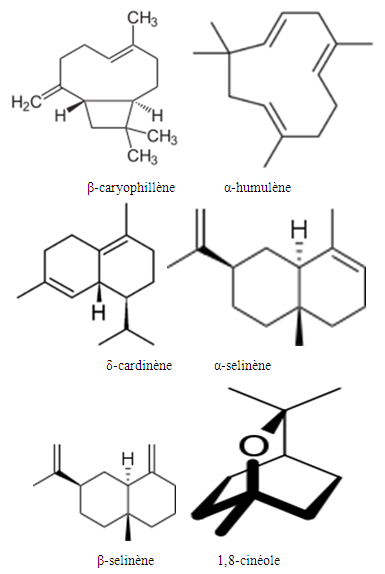 | Figure 2 |
3.4. Antimicrobial Test Results with Tsihanimposa
|
4. Discussion
- The plant Zanthoxylum Tsihanimposa is used by 12 Fonkotany in the commune of Antranokarany to heal the different diseases. But during the measles pandemic the population is using it as a cure for measles. The population uses the planet without knowing the different types of molecules in the plant. It is able to classify the different plants capable of curing diseases thanks to ancestral knowledge. In this plant the rate (E) -β-caryophyllene is 29.56% followed by 1,8-cineole 11.47% and α-humulene 7.28%, There are also other undetermined molecules. In view of the effectiveness of the plant Zanthoxylum Tsihanimposapar compared to other plants, the molecules have a high level are elements able to rid measles or other undetermined molecules. The answer is not clear, so we need further study. But, at the bacterial test level, 7 out of 7 bacteria tested are sensitive to the Zanthoxylum Tsihanimposa plant plus a Candida albicans fungus. Only medicine capable of curing measles regardless of the type of pharmaceutical drug or medicinal plant used in Madagascar even in the world. The plant Zanthoxylum Tsihanimposa is a plant endemic to Madagascar.
5. Conclusions
- Our research leads to a conclusion of the importance of conservation of the different biodiversity in Madagascar. The effectiveness of medicinal plants in the life of the Sakalava Bemazava population indicates that the Zanthoxylum Tsihanimposa plant plays an important role in the face of types of diseases such as diabetes, malaria and especially measles. It is very useful in the treatment of measles that is currently used. The plant in composition with different phytochemicals such as alkaloids (primary, secondary, tertiary), flavonoids, tannins, polyphenols, triterpenes and polysaccharides. On the one hand, it contains molecules such as β-caryophyllene 29.56%, 1,8-cineole 11.47%, α-humilene 7.28%, caryophyllene oxide 3.98% and limonene 1.97%. Zanthoxylum oil inhibits the growth of bacteria such as coliform bacteria, Escherichia coli, Flavobacterium, Actinetobacterium. Zanthoxylum Tsihanimposa is endemic to Madagascar, given its heritage and medicinal value, it should precisely be protected, especially disasters such as deforestation, clearing, bush fires on the island that are most likely to disappear medicinal plants in our forests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML