-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2019; 9(1): 14-18
doi:10.5923/j.plant.20190901.03

Zinc Amelioration of Cadmium Cytogenetic Disorder during Germination of Wheat (Triticum aestivum L.) Grains
Eman F. A. Awad-Allah1, Mohamed A. El-Seehy2, Ibrahim H. Elsokkary1, Abdel Salam A. Abdel Salam1
1Soil and Water Sciences Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
2Genetics Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Correspondence to: Eman F. A. Awad-Allah, Soil and Water Sciences Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

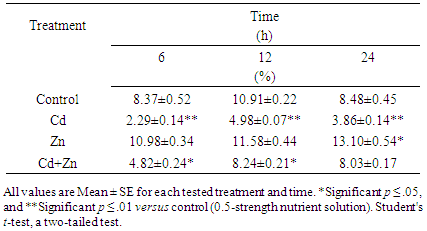
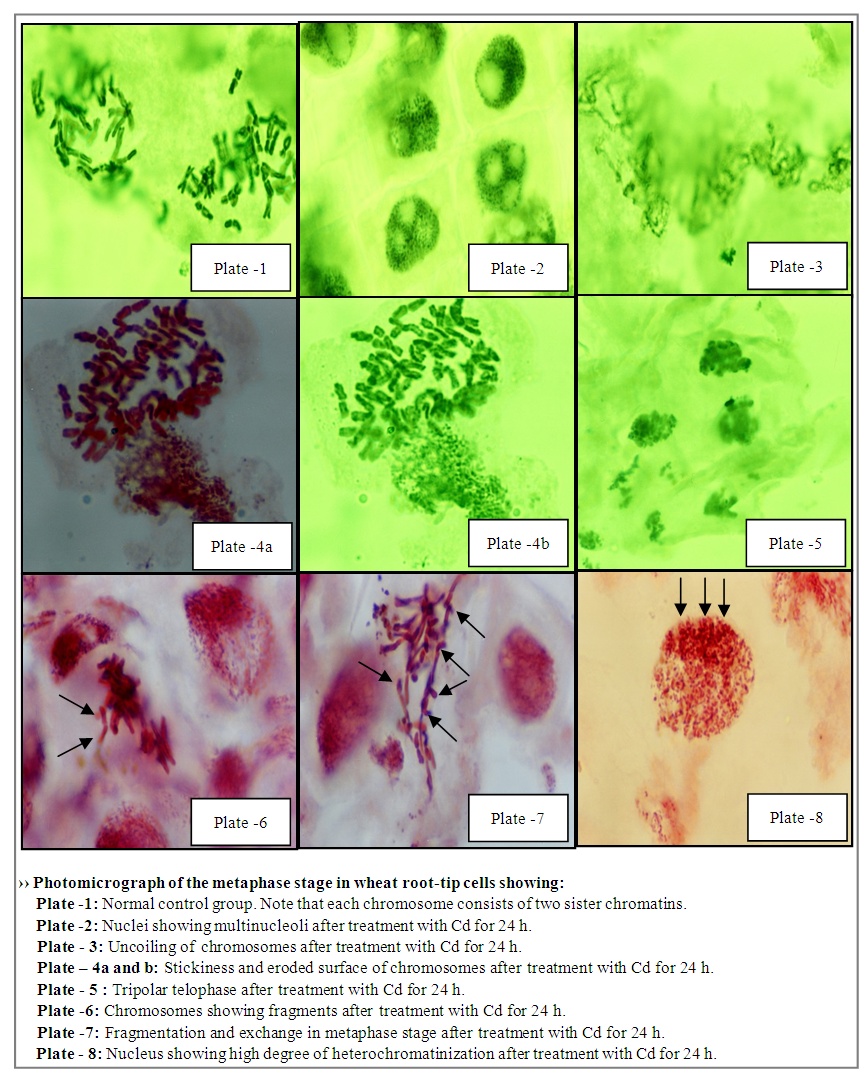
Cytogenetic studies were carried out to investigate the effect of zinc and cadmium, either alone or in combination, on mitotic activity and chromosomal aberrations during the germination of wheat (Triticum aestivum L.) grains after treatment for 6, 12, and 24 h. Cadmium-treated wheat roots had a mitotic index of 2.29, 4.98 and 3.86% after 6, 12 and 24 h, respectively, compared to 8.37, 10.91 and 8.48% for the negative control. Zinc-treated roots had a mitotic index of 10.98, 11.58 and 13.10% after 6, 12 and 24 h, respectively. As these values were greater than those of the control plants, Zn is very effective in causing significant increases in cell proliferation. This is evident with Cd + Zn treatment, which resulted in mitotic proliferation indices of 4.82, 8.24, and 8.03% after 6, 12, and 24 h of treatment. These values are higher than those of Cd-only treatments, suggesting that Zn minimizes the cellular toxicity of Cd. The normal metaphase stage of wheat root-tip cells (i.e., control) showed that each chromosome consists of two sister chromatids. Cytological examination revealed that Cd causes chromosomal damage, as eroded chromosomal surfaces were observed. In addition, cytological examination revealed that fragments were obtained after treatment with Cd and a positive clastogenic effect was achieved. These data reveal that Cd was capable in interfering with chromosomes.
Keywords: Zinc, Cadmium, Mitotic activity, Chromosomal aberrations, Wheat plants
Cite this paper: Eman F. A. Awad-Allah, Mohamed A. El-Seehy, Ibrahim H. Elsokkary, Abdel Salam A. Abdel Salam, Zinc Amelioration of Cadmium Cytogenetic Disorder during Germination of Wheat (Triticum aestivum L.) Grains, International Journal of Plant Research, Vol. 9 No. 1, 2019, pp. 14-18. doi: 10.5923/j.plant.20190901.03.
Article Outline
1. Introduction
- Zinc is an essential plant nutrient involved in several aspects of biochemical reactions, including metabolism and reproductive development. Cadmium is a non-essential plant nutrient that is also highly toxic and can be absorbed readily by plants and accumulates in plant tissues. With the addition of Cd-containing manures, fertilizers, sewage, biosolids and other supplements, Cd contamination is occurring in many agricultural soils. Despite the physicochemical similarity between Zn and Cd, their accumulation, in different plant organs is not similar [1].A Zn-Cd interaction is somewhat controversial, as there have been reports of both antagonism and synergism between the two elements in uptake processes. [2] found high Cd accumulation in the roots of plants with high Zn levels and low solution pH. The ratio of Cd to Zn in plant media controls the occurrence of synergism and antagonism between the two cations [3]. One of the factors affecting Cd uptake by plants is the nutritional status with respect to Zn [4]. Generally, Zn application decreases Cd uptake and accumulation in plants [5]. [6] attributed the competitive interaction to the existence of a common transport system on the plasma membrane. Zn interferes with phloem-mediated Cd transport in durum wheat, possibly by competing with the Cd binding sites of a common transport protein on the plasma membrane of sieve tube cells [7], [4]. Comparative studies of the effect of zinc and cadmium have been carried out regarding the growth characteristics of wheat plant grown on sand culture [8]. Appropriate supply of Zn can enhance plant growth compared to plant growth without the addition of Zn, and can reduce the inhibition of plant growth due to Cd, probably by alleviating Cd-induced damage to plant cells. In contrast, the adverse effects of Cd are due to its interference with several biochemical reactions in plants [9], [10], [11], such as a reduction of cell size and intercellular space [12]. Cytotoxicity of cadmium has been observed to interfere with nucleolar structure and in inhibition of RNA synthesis in the slime mold Physarum polycephalum. A continuum of nucleolar aberrations develops with increasing concentration and duration of Cd exposure, progressing from eccentric nucleoli and multiple bodies to ring-shaped nucleoli [13].[14] studied root tip cells of Allium cepa treated 0.5 μM cadmium chloride (CdCl2). The Cytological effects of cadmium were examined, showing the inhibitory effect of cadmium chloride on the mitotic activity of root tip cells of Allium cepa and cadmium has expressed chromosome aberrations, phyto-and genotoxic activity. Further studies on the effect of Cd and Zn will help in elucidate the regulation mechanisms of rRNA synthesis and/or a cell defense mechanism against protein poisons. Despite increasing knowledge about the diverse effects of Cd on the biochemical processes in plants, there are still prospects for further research into the cytotoxic action of metal ions, comparative characteristics of chromosomal aberration frequency, and number of aberrations per aberrant cell. Therefore, the objectives of this study were to assess the effect of Zn and Cd, both alone and in combination, on cytogenetic root disorder, chromosomal damage, and cellular toxicity.
2. Materials and Methods
- This Cytogenetic study aimed to disclose the capability of cadmium in inducing chromosomal damage and cellular toxicity. The effects of treatment with Cd (as CdCl2) and Zn (as ZnSO4.7H2O) alone or in combination for 6, 12, and 24 h were investigated. Wheat (Triticum aestivum L.) variety Giza 168 grains were soaked overnight in water, and then transferred onto moisture filter paper in a Petri dish and allowed to germinate at 22± 2°C. When roots reached 0.5 cm length, seedlings were transferred to a Petri dish and treated with half-strength nutrient solution containing 0.04 mg Cd/L, 0.100 mg Zn/L, 0.04 mg Cd/L+0.100 mg Zn/L for 6, 12, or 24 h. The negative control groups were allowed to grow on half-strength nutrient solution of Hoagland and Arnon [15] free of Cd and/or Zn.After treatment, seedlings were washed and transferred to 0.05% colchicin solution for 3 hours, then transferred to glacial acetic acid for 1 hour [16], and finally transferred to Carnoy’s solution (consisting of 3 parts absolute alcohol to 1 part glacial acetic acid) for 24 h. The seedling were transferred to 70% ethylalcohol and kept in a refrigerator until use. The obtained data were analyzed statistically, using SPSS version 20.0 [17]. In order to investigate the mitotic chromosomes, the well-known aceto-orcein procedure was used. Metaphases were carefully examined for chromosomal aberrations, such as breaks, gaps, and fragments. To examine the effect of the different treatments on spindle fibers, polyploid cell were counted. To investigate cell proliferation (i.e., mitotic activity), treatment with colchicin was omitted. The mitotic index (MI) was estimated according to the following formula:MI (%) = [No. dividing cells]/[Total no. examined cells] x 100
3. Results and Discussion
3.1. Cytogenetic Effects of Cd and Zn
- This part of the study investigates the clastogenic activity of Cd2+ and disclastogenic of Zn2+ on the wheat genome. The results indicate that Cd2+ was effective at reducing the mitotic activity (Table 1 and Figure 1). In a few instances, the MI for cells treated Zn2+ was equal or greater than that of the control group. It seems probable that this observation is due to cell cycle variations in root meristems. Root meristems are comprised of proliferative and non-proliferative cells [18] that can be categorized into cells that proliferate continuously and cells that proliferate discontinuously or halt temporarily in one or more cycle periods. The root tip cells of Pisum sativum L. after exposure to Cd2+ at concentrations ranging from 0.25 to 250 μM had a reduction of root growth, which is directly related to reduction of apex length, mitotic activity and percentage of DNA-synthetizing cells [19]. Cd2+ has been reported to have the most depression effects on the growth rate of rice seedling growth [20], [21]. A previous study on the toxicity of Cd demonstrated that 1.5 x 10–3 M CdSO4 severely modified nuclear function and structure during division in Physarum polycephalum [13].
|
 | Figure 1. Comparison of the mitotic indices of wheat root tip cells after 6, 12, and 24 h treatment |
3.2. Chromosomal Aberrations
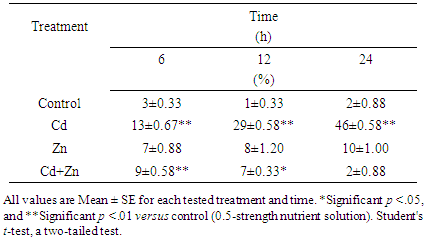
- Cytological examination revealed that cadmium is capable of causing chromosomal damage, as stickiness was observed (Plate- 4a-b). The stickiness reveals that cadmium was very effective at causing alterations of the net charge of the DNA and/ or chromosomal proteins. This conclusion was confirmed by the eroded surface of chromosomes after treatment with cadmium for different durations. The normal metaphase stage in the negative control group of wheat root-tip cells showed that each chromosome consists of two sister chromatins (Plate-1).Multinucleoli were observed (Plate-2), and reflect cellular and molecular toxicity, as the newly synthesized ribosomal RNA precursors accumulated and became associated with the ribosomal proteins. Accordingly, this might lead to large and/or many nucleoli. Tripolar anaphase and metaphase stages were observed (Plate-5), and that Tripolar telophase observed in this study showed that cadmium is capable of interfering with spindle fibers. Plate -3 shows uncoiling of chromosomes after treatment with Cd for 24 h, whereas Plate-7 shows fragmentation and exchange during metaphase stage in wheat root tip cells. Plate-6 shows the fragments obtained after treatment with cadmium for different duration, demonstrating that this element has a positive clastogenic effect on the wheat genome. Heterochromatinization was found at high Cd concentration, giving evidence that the transcriptional activity and cell proliferation decreased after 24 h and that cadmium interfered with spindle fibers (Plate-8). Thus, the present investigation revealed that Cd2+ is capable of interfering with chromosomes, and a positive clastogenic effect was observed. Concerning cadmium clastogenic activity, conflicting results have been reported in the literature. In higher plants, early reports revealed that cadmium salts are capable of inducing C-mitosis in Allium root-tip cells [22]. However, these types of aberrations were not observed in the present study. Chromosome stickiness in metaphase and lagging chromosomes were observed as a result of stickiness. When root meristems were treated with high doses of Cd2+, the toxic effect was more important than the mutagenic effect. This led to classifying cadmium among the highly toxic but weakly mutagenic agents. The total aberrant metaphases obtained after treatment with cadmium and/or zinc is given in Table 2 Figure 2, demonstrating that cadmium caused high cellular toxicity.
|
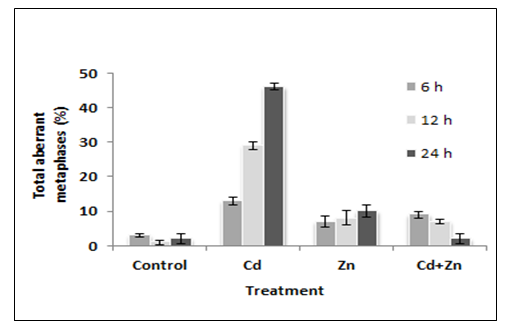 | Figure 2. Chromosomal aberrations induced in root-tip cells after treatment. Error bars represent standard deviation of the mean |
 | Figure 3 |
4. Conclusions
- In conclusion, cadmium-induced chromosome damage in wheat grains can be ameliorated by zinc treatment. A repair effect of zinc was capable of inducing significant increases in cell proliferation and reduced different types of chromosomal aberrations compared to the effect of cadmium.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML