-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2019; 9(1): 8-13
doi:10.5923/j.plant.20190901.02

Evaluation of Crude Extracts of Trametes versicolor (L.:Fr.) Pilát to Control of Phytopathogenic Fungi
Ernesto Hernández Mendieta1, Marcelo Acosta Ramos2, Antonio Segura Miranda2, Catalina Rubio Granados3
1Investigador-Estancia Posdoctoral, Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Chapingo, Estado de México, México
2Profesor Investigador, Programa de Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Chapingo, Estado de México, México
3Investigación y Desarrollo, Grand Mend México, Netzahualcóyotl 214, Texcoco, Estado de México, México
Correspondence to: Ernesto Hernández Mendieta, Investigador-Estancia Posdoctoral, Maestría en Ciencias en Protección Vegetal, Universidad Autónoma Chapingo, Chapingo, Estado de México, México.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The antimicrobial activity of crude extracts of Trametes versicolor was evaluated to control of phytophatogenic fungi using as solvents Water, Hexane, Ethanol, Dichloromethane and Acetone. Concentration of 0, 10, 20, 30 and 50% (v/v) of each extract were evaluated on PDA culture medium using filter paper disks of 6 mm of diam with 60 µl of each concentration. The diffusion area between disks with each concentration of extracts and the fungi growth was recorded at 48, 72, 96, 120, 144 and 166 h after incubation. The extracts obtained from the Hexane and Dichloromethane solvents at concentration of 20, 30 and 50% showed the highest inhibition of the development of Rhizoctonia solani, Fusarium moniliforme, Botrytis cinerea, Cercospora capsici and Alternaria solani above Ethanol and Acetone while the aqueous extract was the least efficient in any of the concentrations evaluated.
Keywords: Trametes versicolor, Crude extracts, Antimicrobial activity
Cite this paper: Ernesto Hernández Mendieta, Marcelo Acosta Ramos, Antonio Segura Miranda, Catalina Rubio Granados, Evaluation of Crude Extracts of Trametes versicolor (L.:Fr.) Pilát to Control of Phytopathogenic Fungi, International Journal of Plant Research, Vol. 9 No. 1, 2019, pp. 8-13. doi: 10.5923/j.plant.20190901.02.
Article Outline
1. Introduction
- Fungi are organisms that obtain their nutrition by absorbing organic matter [1], belonging to a diverse kingdom of which very little has been scientifically described to date [2]. The structural diversity of fungi ranges from unicellular yeast to upper multicellular fungi. A characteristic of these organisms in general is the presence of chitin in their cell wall and just as animals are heterotrophs, that is, they can not synthesize their own food, which they make through hyphae by secretion of digestive enzymes [3].Natural products have the potential to control biological agents that cause various diseases both in humans and in cultivated plants. The increased resistance of bacteria to antibiotics has generated an interest in studying alternative antimicrobial substances for their control [4]. Fungal plant pathogens are among the most important factors that cause serious losses to agricultural products every year. Biological control of plant diseases including fungal pathogens has been considered a viable alternative method to chemical control. In plant pathology, the term biocontrol applies to the use of microbial antagonists to suppress diseases. Throughout their lifecycle, plants and pathogens interact with a wide variety of organisms [5].These interactions can significantly affect plant health in various ways. Different mode of actions of biocontrol-active microorganisms in controlling fungal plant diseases include hyperparasitism, predation, antibiosis, cross protection, competition for site and nutrient and induced resistance [5]. Macromycetes are cosmopolitan heterotrophic organisms that play an important role in the cycle of nutrients and carbon fixation, which maintains a healthy state of the forests in addition to the medicinal and nutritional importance they possess. They grow in the soil or vegetable waste as saprophytes degrading wood and / or in symbiotic associations through the roots of their hosts. They play an important role in the recycling of nutrients, growth and establishment of seedlings in forest soils and are an indicator of the health and maturity of the forest [6].In addition to the bio-environmental importance they possess, macromycetes are also useful as a food and therapeutic tool for the prevention of diseases such as hypertension, hypocholesterolemia and cancer [7-11], besides offer an effect of control of microorganisms such as Bipolaris sorokiniana, Fusarium culmorum, Gaeumannomyces graminis var tritici, Rhizoctonia solani, Pyricularia orysae, Pseudomonas aeruginosa, Staphyloccus aureus, Bacillus megaterium, Staphylococcus aureus, Escherichia coli, Candida albicans and Candida glabrata, among others, in which the species Trametes versicolor, Pleurotus eryngii var. Ferulae, Lentinula edodes, Coriolus versicolor, Flammulina velutipes, Ganoderma lucidum, Hypholoma fasciculare, H. sublateritium, Kühneromyces mutabilis, Lentinula edodes, Lentinus tigrinus, Pholiota alnicola, Ph. Aurivella, Ph. Destruens, Pleurotus ostreatus, P. cornucopiae, Polyporus squamosus, P. subarcularius, P. varius and Schizophyllum commune [12-14].Trametes versicolor is probably the most studied and biologically evaluated macromiceto of all the fungi in this group. These studies focus exclusively on two polysaccharides -Krestin PSK and polysaccharide peptides (PSP) -, which are heteroglycans with fixed glycoside components α- (1-4) and β- (1-3), with a protein or poly peptide component [15,16] that confer properties of stimulation of immune system [16], as well as anticarcinogenic properties [18] and antioxidants [19] in human beings [20,21]. However, studies have also been carried out on various species of bacteria and fungi with the aim of reducing the excessive use of agrochemicals, reducing the risks of generating resistance to antibiotics and producing foods with a high degree of innocuousness. In this regard, Machoa and Tanata [13] when evaluating aqueous extracts of this macromiceate report an antibacterial effect on 60% of the strains used of Pseudomonas aeruginosa and 20% of the strains of Staphyloccus aureus; while the ethanolic extract showed an effect of 40% on the strains of P. aeruginosa concluding that the type of solvent is directly related to the effectiveness detected. Aqueous extracts of Pholiota nameko showed a higher activity on Staphylococcus aureus; while the ethyl acetate extract does not show activity [22]; On the other hand, the ethanolic extract of Ganoderma lucidum has a greater antimicrobial effect than the aqueous extract of Staphylococcus aureus, which is possibly attributed to the extraction method, to the solubility of the secondary metabolites, as well as to the polarity of the fungal extracts [23]. The secondary metabolites produced by fungi are synthesized in greater amounts in apolar extracts, since they have a high affinity to low polarity solvents being rich in biologically active terpenoids, substances to which a large part of the antimicrobial and fungal activity is attributed [24]. With the purpose of developing biological strategies for the control of phytopathogenic fungi, the present study was conducted with the objective of evaluating the antagonistic activity in vitro of crude extracts of Trametes versicolor strain Mo008 againts Rhizoctonia solani, Fusarium moniliforme, Botrytis cinerea, Cercospora capsici and Alternaria solani using water, hexane, Ethanol, dichloromethane and acetone as solvents.
2. Materials and Methods
2.1. Phytopathogens
- Strains of pathogenic fungi that were used in this research were provided by Strain Collection of Phytopathology Area from Master’s Program of Crop Protection in Chapingo University.
2.2. Preparation of Crude Extracts
- The solvents used for the preparation of the extracts were water, hexane, ethanol, dichloromethane and acetone. For each of these, 25 g of Malt Agar Extract culture medium (Difco®) was weighed with the development of Trametes versicolor strain Mo008 for 30 days of growth and cut into pieces in a mortar. To the mortar was added 20 ml of Liquid Nitrogen and macerated until a homogeneous paste was obtained. Subsequently, the mash was transferred to a 250 ml ground-glass flask and 200 ml of solvent were added. The solvent flasks were placed on an oscillator for 72 h at 100 rpm at lab temperature. Subsequently, the macerate was separated from the solvent by vacuum filtration into a sterilized flask of the same volume and the solvent was removed by reduced pressure at 55 °C in a roto-steam at 100 rpm (Rotavapor® R-300), doing this for solvents hexane, ethanol, dichloromethane and acetone; while the water-based extract was used directly from that obtained from filtration. After separating each of the solvents, 100 ml of sterile bidistilled water was added to each flask, rinsed thoroughly and the water with the extract was recovered in 150 ml amber bottles and stored in total darkness at 10 °C for its subsequent employment.
2.3. Concentrations of Crude Extracts
- A 10 ml solution in sterile distilled water with concentrations of 0, 10, 20, 30 and 50% was prepared for each of the extracts in pre-sterilized screw-cap test tubes.
2.4. Preparation of Sensitivity Discs
- 6 mm diameter filter paper discs (Whatman®) were made, numbering from 1 to 5 where 1 is 0% and 5 is 50% concentration of crude extrat and then sterilized.
2.5. Evaluation of Biological Effectiveness
- The biological effectiveness of the extracts was determined based on the length of the mycelium developed in the Petri dish of 10 cm of diameter with PDA culture medium (BD Bioxon®), using strains of Rhizoctonia solani, Fusarium moniliforme, Botrytis cinerea, Cercospora capsici and Alternaria solani of 7 days of growth. 6 mm diameter agar discs were used from each strains which were placed in the center of the Petri dish with culture medium and each filter paper disc impregnated with 60 μl of each of the concentrations per extract was placed equidistant way in the petri dish 3 cm from the center. The Petri dish were incubated at 25°C with 12 h of light / dark. The distance in mm of the micelium growth was recorded from the edge of the filter paper to the growth edge of each fungus at 48, 72, 96, 120, 144 and 166 h after incubation. The results were analyzed under a completely randomized factorial design with 10 repetitions, with 3 factors and 5 levels per factor, applying the analysis of variance and Tukey's mean comparison test with α = 0.05 with the statistical analysis software. SAS®.
3. Results and Discussion
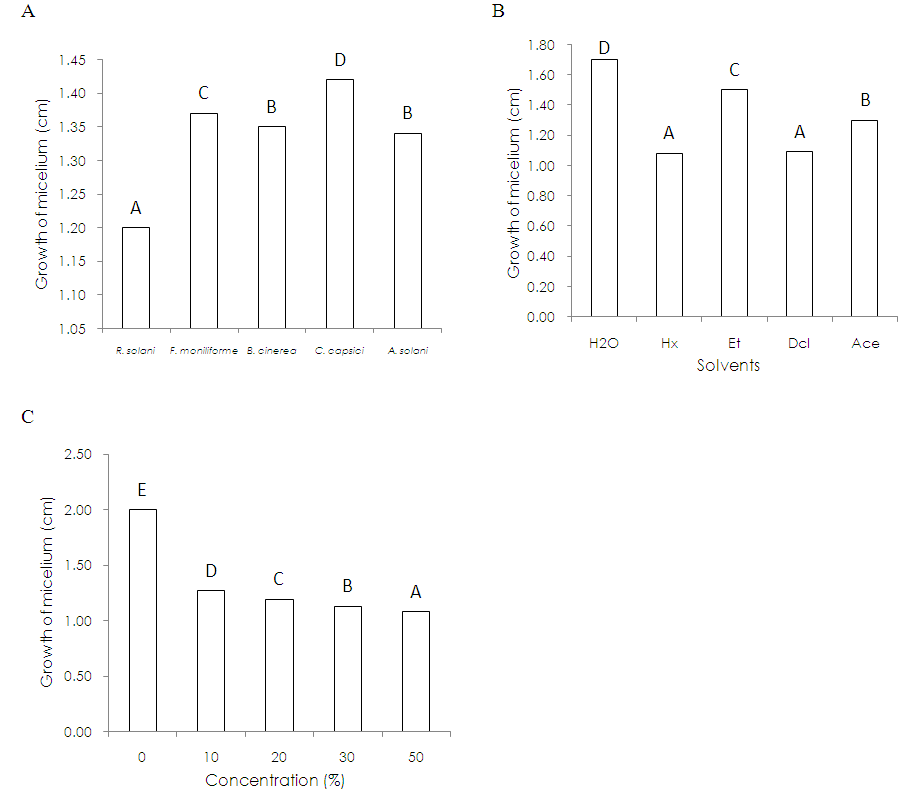
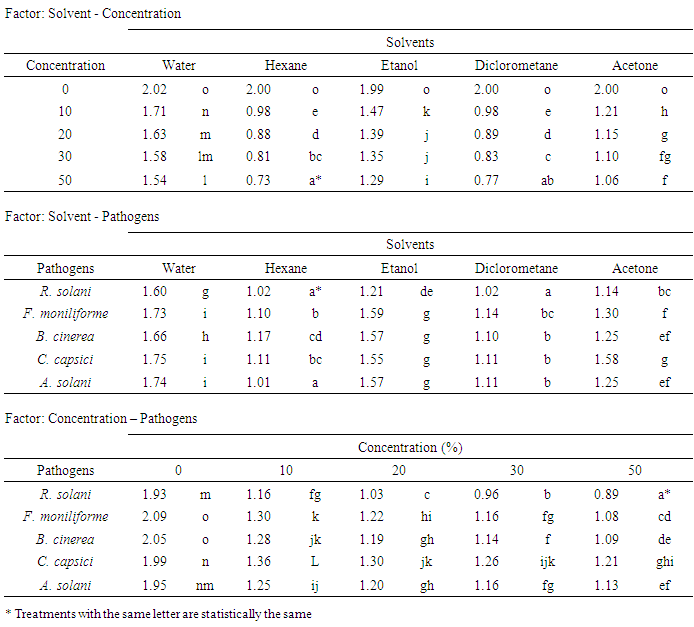
- The in vitro anti fungal activity of Trametes versicolor strain Mo008 evaluated through the control effect that crude extracts showed on the phytopathogenic fungal complex shows that of the strains studied, the most susceptible was Rhizoctonia solani (Figure 1A); while the mycelial development of Botrytis cinerea and Alternaria solani was statistically the same, with Cercospora capsici being the least susceptible fungus followed by Fusarium moniliforme. On the other hand, of the solvents used to obtain the crude extracts, Hexane and Dichloromethane were statistically equal in the inhibition of the development of the mycelium of the strains; while Acetone offered a better result than Ethanol and of all these, the aqueous extract favored the greater growth of phytopathogenic fungi (Figure 1B). Likewise, the studied concentrations of each of the crude extracts evaluated indicate that T. versicolor provides metabolites that are capable of inhibiting the development of microorganisms and the results obtained indicate that the effectiveness of these is directly related to their concentration in the extract (Figure 1C).Hexane and Dichloromethane were the solvents that showed the highest inhibition of mycelial development at a concentration of 50%; presenting significant differences with the concentrations of 0, 10, 20 and 30% as well as with the solvents based on Water, Ethanol and Acetone. Regarding the response of the phytopathogenic fungi to the extracts, Rhizoctonia solani was more susceptible to the Hexane and Dichloromethane solvents and the development of Alternaria solani was also inhibited with the Hexane-based extract. Acetone as a solvent for the formulation of crude extracts of T. versicolor has a greater capacity of inhibition on the development of the fungal complex than Ethanol and the Aqueous extract (Table 1). On the other hand, Fusarium moniliforme, Botrytis cinerea and Cercospora capsici also showed susceptibility to solvents, excelling the concentrations of 20, 30 and 50% in all cases.
|
4. Conclusions
- Trametes versicolor is a basidiomycete that produces secondary metabolites that are washed away by means of water, Hexane, Ethanol, Dichloromethane and Acetone based solvents, which had an inhibitory effect on a complex of phytopathogenic fungi (Rhizoctonia solani, Fusarium moniliforme, Botrytis cinerea, Cercospora capsici and Alternaria solani), excelling in the control of these the crude extracts that used as solvent Hexane and Dichloromethane in concentrations of 20, 30 and 50% (v / v). The crude extracts from the ethanol and acetone solvents also inhibited the development of the fungi studied, the least efficient being the water-based extract in any of its concentrations.
ACKNOWLEDGEMENTS
- To the National Council of Science and Technology (CONACYT) for the support granted to carry out the Postdoctoral Stay, as well as the Master's Program in Crop Protection of the Autonomous University of Chapingo, for the facilities granted to carry out the present investigation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML