-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2018; 8(1): 15-24
doi:10.5923/j.plant.20180801.03

The Use of Extracts from Nigerian Indigenous Plants as Staining Techniques in Bacteriology, Mycology and Histopathology
Nkechi Augustina Olise1, Ifeoma Bessie Enweani2, Ifeanyi Onyema Oshim2, Evelyn Ukamaka Urama2, Clinton Chinedu Ngwoke3
1Department of Medical Laboratory Science, School of Basic Medical Science, University of Benin, Benin-City, Nigeria
2Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Anambra, Nigeria
3Department of Medical Microbiology, Faculty of Medical Laboratory Science, Usman Danfodiyo University Sokoto, Nigeria
Correspondence to: Nkechi Augustina Olise, Department of Medical Laboratory Science, School of Basic Medical Science, University of Benin, Benin-City, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Dyestuffs from Nigerian plants were extracted and their staining ability on some species of bacteria, fungi and tissue biopses were determined. The study was carried out, to demonstrate the potential use of G. kola mesocarp, V. doniana fruit, L. aculaeta fruit, L. inermis leaf, C. ferrugnea fruit and P. soyauxii stem extracts as dyes/stains on Escherischia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Candida albicans, Aspergillus niger and human appendix tissue. Fully automated Soxhlet solvent extraction technique was used for the extraction using ethanol and methanol. Solvent-Solvent fractionation technique was also, done to obtain purer form of the plants extracts using Ethylacetate, N-hexane and N-butanol as solvent. The simple stains like neutral red, lactophenol cotton blue and Haematoxylin & Eosin were used as positive controls for staining bacterial, fungi morphology and human appendix tissues respectively. The dyestuff showed stronger staining affinity for fungi than bacteria and tissue biopses. The extracts of C. ferruginea and P. soyauxii treated with inorganic acid, (Hydrochloric acid) increased the intensity of their colours from red to deep red colour. In the use of plant extracts as stains\dyes on clinical bacterial isolates, fungal and tissue cells, the pH of the six plants extracts ranged from 4.2 to 6.4. All extracts had acidic pH. The dyestuff prepared from G. kola mesocarp was not soluble in water but was only soluble in organic solvent (methanol and ethanol). V. doniana fruit extract was soluble in water but colour was not fast. L. aculata seed extract did not yield significant colour during extraction with organic solvent as well as with water. L. inemis was moderately soluble in water and more soluble in organic solvent (methanol) although after one week extract appear dark brown. The chemistry behind the colour was not understood. C. ferruginea fruit extract was slightly soluble in water and more soluble in organic solvent (ethanol). Pterocarpus soyauxii dissolved poorly in water probably because of the oil base nature but was very soluble in organic solvent like methanol. Alcohol extract of four plants namely: G. kola, L. inermis, C. ferruginea, and P. soyauxii stained Gram- negative bacteria poorly without proper differentiation because a suitable accentuator was not discovered in this study to enhance their staining pattern. Possible findings from the use of study extracts as stains provide useful alternative that would be readily accessible and cheap which can translate to lower cost of laboratory stains for better management of patients. The results obtained indicate that extracts with good staining ability have the potential for use in the morphological identification of moulds, yeast cells, human appendix tissues and not only used in diagnostic microbiology but also in histology for future studies.
Keywords: Staining, Plant extracts, Bacteria, Fungi and Tissue biopses
Cite this paper: Nkechi Augustina Olise, Ifeoma Bessie Enweani, Ifeanyi Onyema Oshim, Evelyn Ukamaka Urama, Clinton Chinedu Ngwoke, The Use of Extracts from Nigerian Indigenous Plants as Staining Techniques in Bacteriology, Mycology and Histopathology, International Journal of Plant Research, Vol. 8 No. 1, 2018, pp. 15-24. doi: 10.5923/j.plant.20180801.03.
1. Introduction
- Dyes are chemical substances of chemical or synthetic origin, soluble in a medium used to impart a desired colour to a non food material like paper, leather, wood, textile and even cosmetics in a process known as dying [1] Pigments are the specific chemical compounds responsible for the visible colour in the plant parts [1]. [2] reported that bacterial cells are attracted to the opposite charge ions of the stain. In histopathology, the most commonly used dye is haematoxylin obtained from a South African tree known as logwood (Haematoxylium campechianum. L). Studies conducted by [3] have shown that the red dyestuff obtained from Pterocarpus osun species was used in staining tissue section for histopathological diagnosis of diseases. Microbial stains are used to impart colour in order to make the cells and tissues more distinct [4]. [5] are of the opinion that the pH of the dye may alter staining effectiveness since the nature and degree of the charge on cell component changes with pH. Most of the dyes used for bacterial and fungal smears are synthetic [6]. However, synthetic dyes cause skin allergies and other harms to human body on exposure and produce toxic waste [7]. Research has shown that extracts obtained from natural sources such as plants, animal, vegetable sources, insects and soil hold promise as a potential source of cheaper stains [8], and this paves way in the discovery of these medicinal plants used in this study which include; Garcinia kola, Vitex doniana, Lantana aculeate, Lawsonia inermis, Cnestis ferruginea and Pterocarpus soyauxii. In this report, the staining potentials of these indigenous Nigerian plant extracts were determined using selected bacteria, yeast cells, moulds and tissue biopses. The effectiveness of the extracts was also compared to conventional staining reagents.
2. Materials and Methods
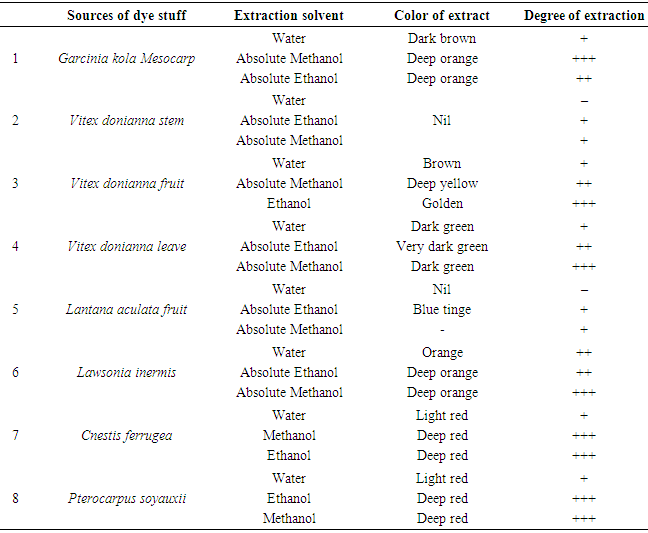
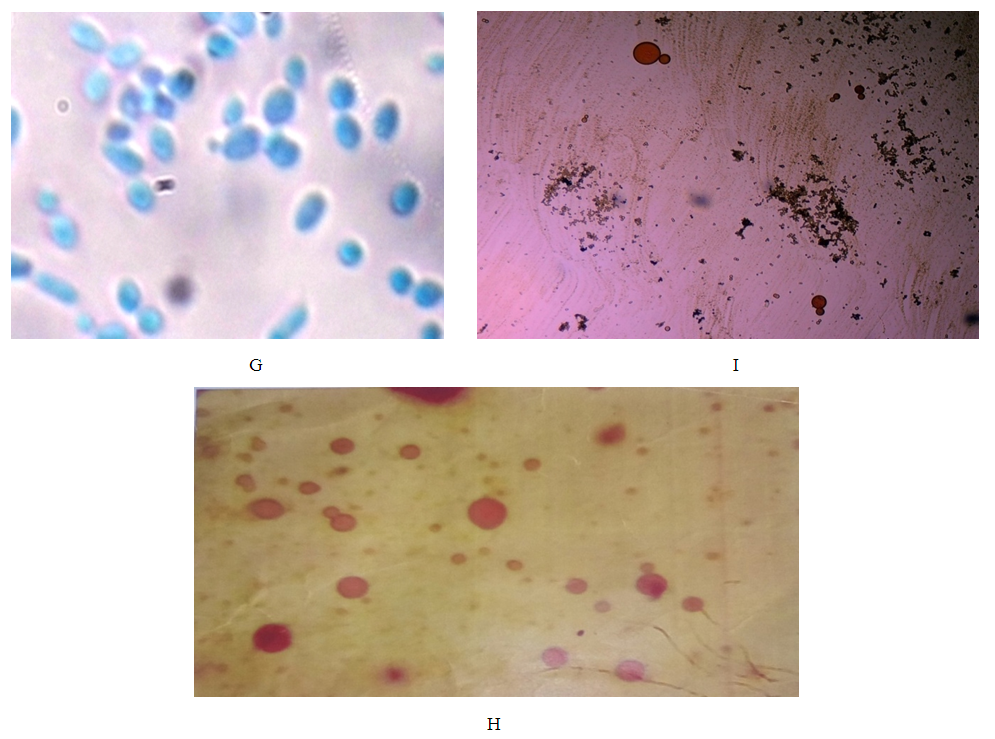
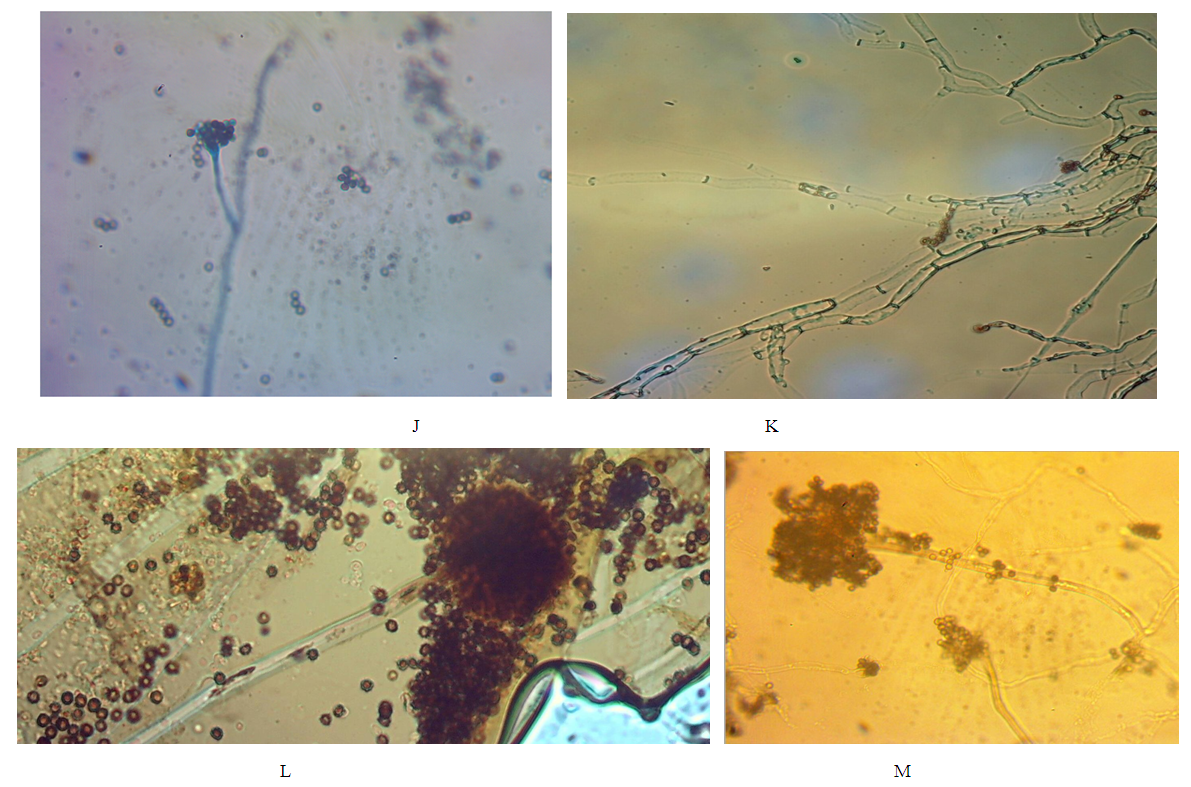
- Design of studyRandom sampling was adopted for this study because of the geographical locations and distributions of the different plants.Collection of PlantsSix indigenous plants were used which were collected across three different states in the Southern region of Nigeria, namely: Edo, Delta and Anambra states. The plants are Garcinia kola, Vitex doniana, Lantana aculaeta, Lawsonia inermis, Cnestis ferruginea and Pterocarpus soyauxii. The six plants were collected as follows: Lawsonia inermis leaf and Lantana aculata leaf were collected in Edo State, Cnestis ferruginea fruit was obtained from Delta State, Garcinia kola fruit and Pterocarpus soyauxii stem, Vitex doniana leaf, stem and fruit were collected from Anambra State.Plant Identification & AuthenticationPlants collected were identified authenticated by Prof J, F Bamidele and Dr H, A Akinibosun plant Taxonomists using their local names and standard texts. Samples of plants were deposited in the herbarium of the Department of Plant Biology and Biotechnology University of Benin. Their Voucher numbers are as follows: UBH365 (Garcinia kola), UBH366 (Vitex doniana), UBH367 (Lantana aculata), UBH368 (Lawsonia inermis), UBH369 (Cnestis ferruginea) and UBH370 (Pterocarpus soyauxii).Study SiteAnalysis on the plants parts was carried out in the Faculty of Phamaceutical Sciences, Agulu, Nnamdi Azikiwe University, Anambra state.Isolation of Bacteria and Fungi from Clinical SamplesIsolates of Monganella monganii, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pneumonia, Proteus mirabilis, Klebsiella pneumoniae Candida albicans and Aspergillus niger were all clinical isolates obtained from the Central Hospital and Stella Obasanjo Hospital Benin City, Edo State. Pure isolates were obtained by culturing on the respective selective media. Biochemical tests were performed to identify and confirm isolates. Fresh plates of test bacteria were made from the isolate cultures obtained on agar slants. Discrete colonies of fresh cultures of the different bacterial isolates were then picked and suspended in 5ml Nutrient broth in well labelled sterile bottles and incubated at 37°C prior to antimicrobial susceptibility testing. The fungi species; C. albicans and A. niger were similarly treated, but cultured on Sabouraud dextrose medium.Ethical ApprovalThe study was approved by the institutional ethics committee at Hospitals Management Board, Benin City, Edo state and Ethical Committee Faculty of Health Sciences (the ethical approval number: HA 577/VOL.11/173). The study design in December, 2017.ExtractionFully automated Soxhlet solvent extraction technique was used for the extraction using ethanol and methanol. The filtrates were evaporated using rotatry evaporator and were finally concenterated to dryness using water bath at a temperature of 50°C [9]. Hospitals Management Board, Benin City, Edo state and Ethical Committee Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Nnewi Campus Nnewi. The crude extract were weighed after extraction, placed in an air-tight and water-proof container and kept in a refrigerator at 4°C.FractionationSolvent-Solvent fractionation technique was done to obtain purer form of the plants extracts using Ethylacetate, N-hexane and N-butanol as solvent [9].Analytical high pressure liquid chromatography (HPLC)The fractions were subjected to analytical HPLC. In the HPLC an efficient separation is achieved by passing a mobile phase through a column using high pressure pumps. Analytical HPLC was used to identify peaks from fractions, and to evaluate the purity of isolated compounds. The different components in the mixture pass through the column at different rates due to differences in their partitioning behaviour between the mobile liquid phase and the stationary phase. The solvent gradient used started with 10:90 (MeOH: nanopure water (adjusted to pH 2 with phosphoric acid) increasing to 100% MeOH in 45 min and run till 60 min. The compounds were detected by an UV-VIS diode array detector. Two miligram (2mg) of each of the dried fractions were reconstituted with 2mL of HPLC grade methanol soncated for ten minunits (10 min), centrifuged and filtered. One hundred microliter (100μL) of the filtrate containing dissolved samples were each transferred into HPLC vials containing 500μL of HPLC grade methanol. HPLC analysis was carried on the samples with a Dionex P580 HPLC system coupled to a photodiode array detector (UVD340S, Dionex Softron GmbH, Germering, Germany). Detection was at 235, 254, 280 and 340 nm. The separation column (125 × 4 mm; length × internal diameter) was prefilled with Eurospher-10 C18 (Knauer, Germany), and a linear gradient of nanopure water (adjusted to pH 2 by addition of formic acid) and methanol was used as eluent.Histological StainingThree sections of human appendix tissues already processed using standard histological procedures and stained with extract of P. soyauxii as follows. Briefly, prepared slides were deparafinized by passing over over a flame from a burner and then placed in xylene. This was done repeated severally until tissue became bare on slide. Tissues were then hydrated by passing through decreasing concentration of alcohol baths and water (100%, 90%, 80%, 70%). Slides were stained with heamatoxylin dye for 4 minutes and washed in running water until section became blue. Tissues were differentiated in 3% acid alcohol for 5 minutes. Section was washed in running water.Slides were counterstained with 1% to 5% of P. soyauxii extract for 5 minutes. Slides were washed in water for 3 minutes and dehydrated in increasing concentration of alcohol and cleared in xylene. The slides were mounted with DPX and observed under the Microscope.Duplicate section of appendix tissue were stained using the conventional Gill, Harris and Mayers Heamatoxylin and Eosin staining Technique.Preparation of Extract of P. soyauxii as counter stainZero point one gram to zero point five gram (0.1g-0.5g) of extract of Pterocarpus soyauxii stem were dissolved differently in 10mls of methanol to form 1% to 5% of extract and 0.2mls of HCL was added to enhance the intensity of the colour of the extract.Preparation of Extract for Staining BacterialLawsonia inermis extract preparation:One gram (1g) of L. inermis leaf extract was reconstituted with ten mililiters (10ml) of distilled water. This was filtered with whatman filter paper and transferred into a universal continer and stored in the fridge at 4°C and is ready for use.G. kola Extract PreparationOne gram (1g) of Garcinia kola mesocarp extract was dissolved in 100mls of 70% ethanol, this was filtered and transferred into a clean universal bottle and stored at 4°C in the fridge and it is ready for use.Cnestis ferruginea fruit extract preparationOne gram (1g) of C. ferruginea fruit extract was dissolved in ten mililiters (10ml) of seventy percent (70%) alcohol and zero point two mililiter (0.2ml) of hydrochloric acid (HCL) added to increase the intensity of the colour. It was filtered with whatman filter paper No 1 and stored in a clean universal bottle and is ready for use.Pterocarpus soyauxii extract preparationOne gram of P. soyauxii extract was dissolved in one hundred mililiters (100ml) of seventy percent (70%) alchohol and zero point two (0.2ml) of hydrochloric acid (HCL) was added to increase the intensity of the colour. This was filttered and transferred into screw capped bottle and is ready for use.Staining of bacteriaSmears of organisms (S. aureus, E. coli, P. aeruginosa, P. mirabilis, K. Pneumoniae) were made on twenty-five sets of clean grease free slide and heat fixed [10, 11]. Gram staining reagents (crystal violet, lugols iodine and acetone) were used except for the counter stain (neutral red). These slides were counter stained with the solutions made from extracts of G. kola mesocarp, Lawsonia inermis leaf, Cnestis ferruginea fruit and Pterocapus soyauxii stem. Control slide was also prepared and stained by Grams method using neutral red as counter stain [12].Staining of FungiA drop from prepared solutions of extracts was placed on clean grease free microscope slides. Fungi (C. albicans and A.niger) grown on Sabouraud dextrose agar (SDA) medium and the cover slips from the slide cultures were placed on a pool of the staining reagents on the slides [13]. The slides were allowed to stand for 3 minutes and the morphology of the organism viewed at X40 magnification. Control slides were prepared and stained with lactophenol cotton blue.
3. Results
- Fully automated Soxhlet solvent extraction technique was used for the extraction of these Nigerian indigenious plants using ethanol and methanol as shown in Table 1.
|
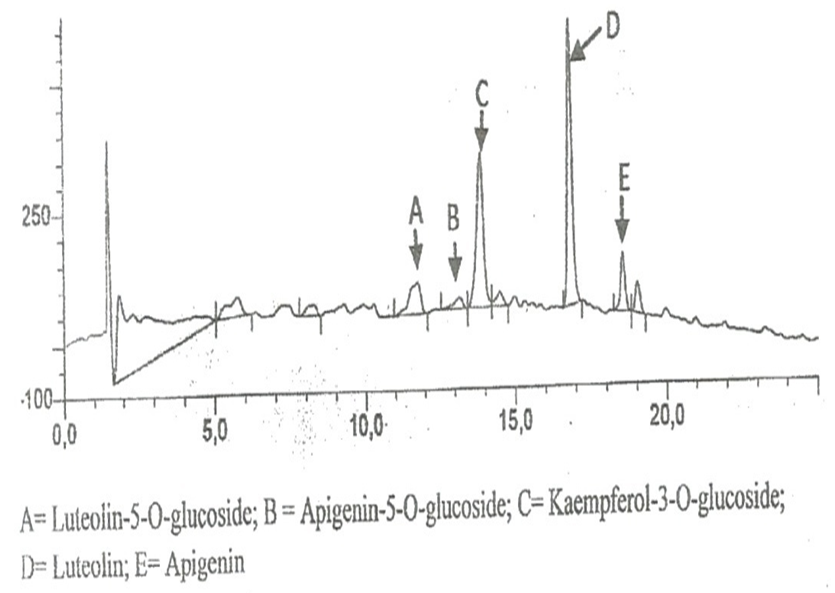 | Figure 1. Components of L inermis on HPLC |
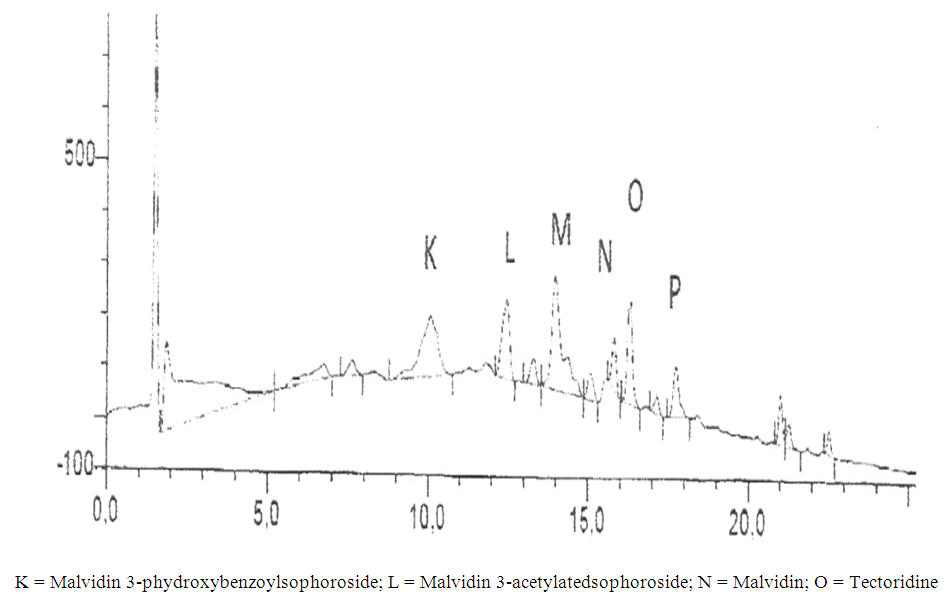 | Figure 2. Components of P. soyauxii on HPLC |
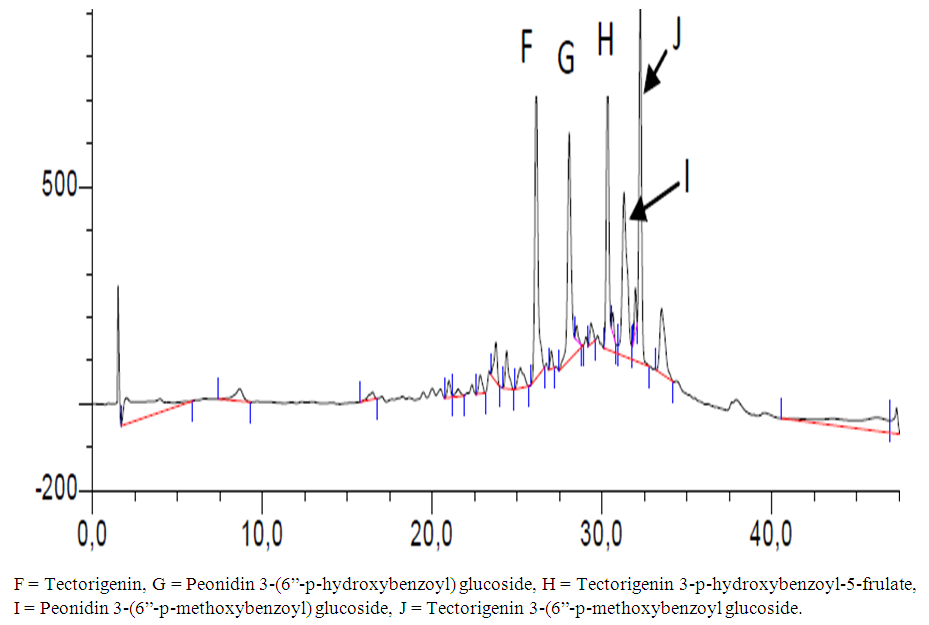 | Figure 3. Components of V. doniana fruit on HPLC |
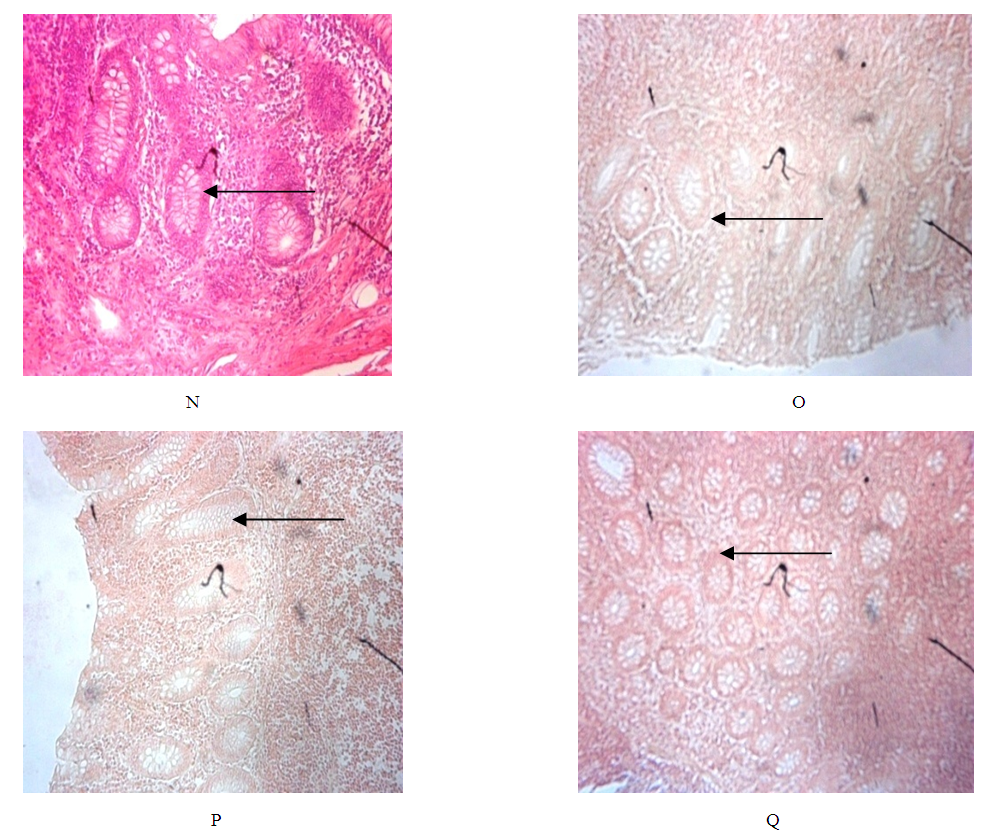
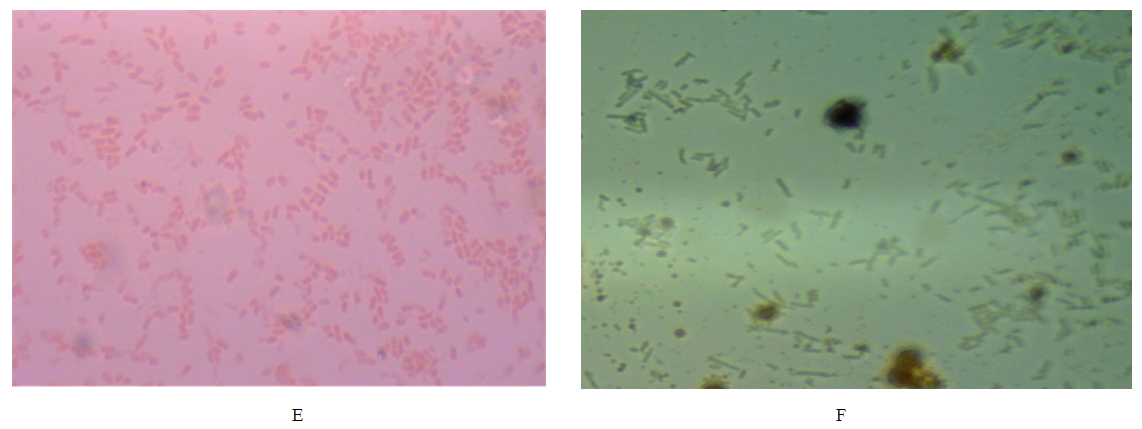 | Plate four E: E. coli stained with grams staining method with neutral red as counter stain, Plate four F: E. coli stained with grams staining method with G. kola as counter stain. |
4. Discussion
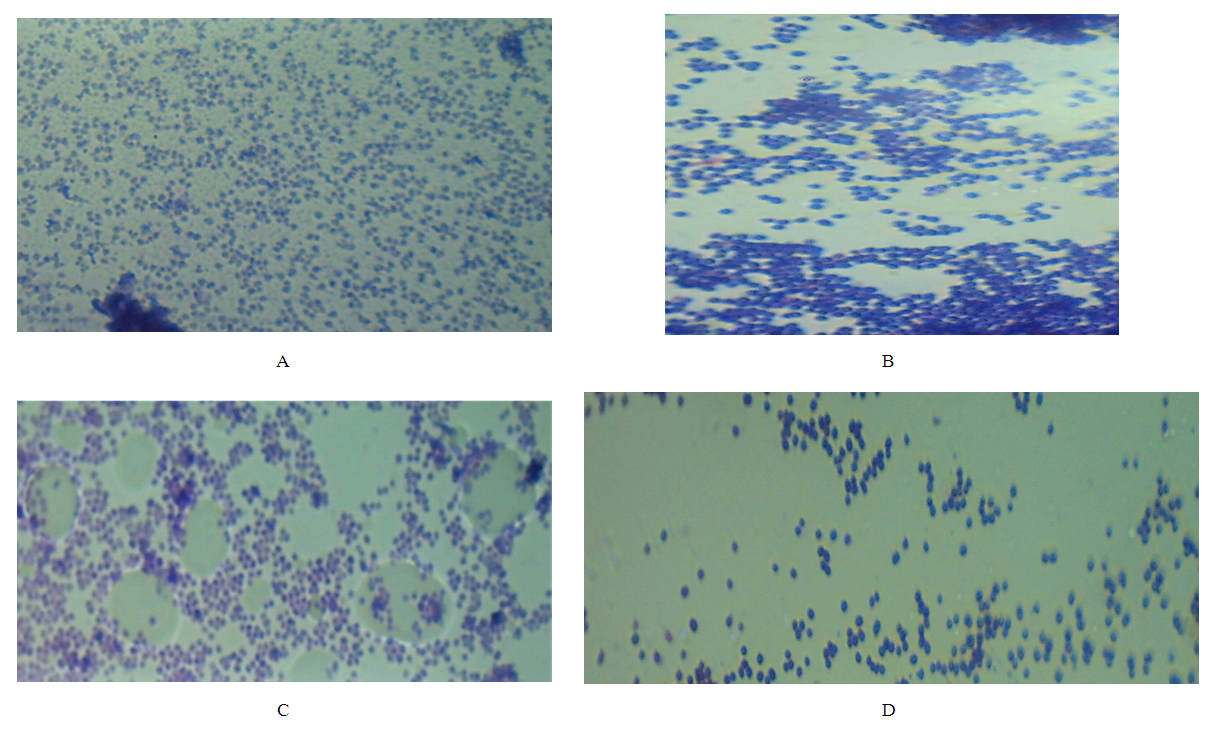
- In the use of plant extracts as stains\dyes on clinical bacterial isolates, fungal and tissue cells, the pH of the six plants extracts ranged from 4.2 to 6.4. All extracts had acidic pH. Extracts with acidic pH have affinity for cytoplasm which is basic in nature. [12] that is why extracts act as counter stains and not primary stains which are basic and so has affinity for the nucleus because of the nuleic acid. The dyestuff prepared from G. kola mesocarp was not soluble in water but was only soluble in organic solvent (methanol and ethanol). This is so because of the high lipid content fifteen point four percent (15.4%) of G. kola mesocarp extract. V. doniana fruit extract was soluble in water but colour was not fast. L aculata seed extract did not yield significant colour during extraction with organic solvent as well as with water. L. inemis was moderately soluble in water and more soluble in organic solvent (methanol) although after one week extract appear dark brown. The chemistry behind the colour was not understood. C. ferruginea fruit extract was slightly soluble in water and more soluble in organic solvent (ethanol). Pterocarpus soyauxii dissolved poorly in water probably because of the oil base nature but was very soluble in organic solvent like methanol. Alcohol extract of four plants namely: G. kola, L. inermis, C. ferruginea, and P. soyauxii stained Gram- negative bacteria poorly without proper differentiation because a suitable accentuator was not discovered in this study to enhance their staining pattern. The poor stain could also be attributed to chemical composition of the stains from extracts and its ability to penetrate the cell walls of the organisms. They can be regarded as simple stains like neutral red and lactophenol cotton blue used for staining bacterial and fungi morphology respectively, without demonstrating internal organnels of the organism.Appendix in control reveals well differentiated staining extending from the muscularis externa through the submucosa to the mucosa. The germinal center and lymphoid follicles appear well differentiated and stained (black arrow). The APP (M), APP (H) and APP (G) show more intense staining at five percent (5%) concentration. The lymphoid follicles are stained and fairly differentiated. The APP (G) gave the best intensified and differentiated staining compared to the APP (M) and (H). In the HPLC analyses of the six different plants part extracts, the ethylacetate solvent fraction of the V.doniana fruit, revealed five compounds which were identified to be Tectorigenin, Peonidin 3–(6-parahydroxybenzyl) glucoside, Tectorigenin–3–p– hydroxybenzyl–5–frulate, Peonidin–3–(6”– p – methoxybenzoyl) glucoside and Tectorigenin –3– (6”–p–methyoxybenzoyl) glucoside (figure 4). These compounds are generally called flavonoids which are divided into six sub groups, of which these five compounds detected fall into two subgroups: namely; Isoflavones and Anthocyanins. Tectorigenin, Tectorigenin (3–p–hydroxybenzoyl–5–frulate) and Tectorigenin 3–(6”–p– methydoxybenxoyl) glycoside are Isoflavones while Peonidin 3–(6”–p–hydroxybenzol) glucoside and Peonidin 3(6”– p– methoybenzoyl) glucoside are Anthocyanins. Isoflavones are found in a class of plants known as phytoestrogens because of their similar chemical structure and function to the female sex hormone estrogen [14]. Vitex doniana fruit is rich in isoflavones and the three types of isoflavones discovered have 13.15%, 11.70% and 14.62% respectively as peak area.Isoflavones are widely appreciated and are currently the subject of intense research and discussion, this is because it protects against hormone related disorders such as breast cancer prostate cancer, osteosarocoma, lung carcinoma, and ovarian cancer. [14-16]. Anthocyanins are polyphenols and generally accepted as the most important group of water soluble pigment in nature [17]. They are responsible for the blue, purple, red or orange colour of many fruits and vegetables [17]. They are distinguished from other flavonoids due to their capacity to form flavylium cations [18]. One of them is Peonidin found in this study which is responsible for the colour found in V.doniana fruit (Purplish blue colour) and this is also influenced by the abundance of hydroxyl group. The hydroxyl is responsible for the bluish shade while the methoxyl influence the redish colour [19, 20]. Anthocyanins can exert a major chemopreventive activity due to their antioxidant property [21] by scavenging reactive oxygen and reactive nitrogen species or by chelating trace metals involved in free radical production [22]. In the analysis of lawsonia inermis leaf extract, the ethylacetate solvent fraction yielded five major compounds identified as Luteolin–5–glucopyranoside, Apigenin–5–0– glucopyranoside, Kaemferol – 3- 0 – glucopyranoside, Luteoline and Apigenin.These five compounds are flavones a type of flavonoid. Leutolin mono glycoside peak area is 5.13% in Lawsonia inermis. Apigenin monoglycosides is also a flavone present in form of glycosides in Lawsonia inermis with peak area of 1.35% concentration. Apigenin suppresses cancer cells, by altering a very specific step in gene regulation making cancer cells to die like normal cells. Apigenin also binds a very important protein called HnRNPA2 and this connection thus inhibit breast cancer cells and so cells die in programmed way {Restors the single splitting of cells instead of double splitting which is a characteristics of breast cancer cells (induces apoptosis). It also has anti-inflammatory properties. It blocks the production of uric acid. It has anti depressant-like effect. Some other sources of Apigenin are found in thyme, peppermint, chamomile herbs, red wine and tomatoes sauce. Kaempferol monoglucoside, this is a flavone, present at a high percentage (peak area) as 14.87% in lawsonia inermis leaf extract, it is a natural flavonol a type of flavonoid, and appear as a yellow crystalline solid, this contributes to the yellow colour exhibited by Lawsonia inermis leaf extract. Kaempherol is also found in apples, grapes, tomatoes, broccoli, cucumbers, letuce, green beans and moringa. It is a strong antioxidant and it combines with quecitin to reduce proliferation of cancer cells [22, 23]. It is a potent promoter of apoptosis [24]. In Chemotherapy it is much less toxic to normal cells in comparison with standard chemotherapy drugs [25]. Luteolin had the highest concentration with peak area of eighteen point seventy-eight (18.78%) in lawsonia inermis leaf HPLC analysis, this forms part of our daily nutrition in a relatively low amount (Less than 1mg/day [25] It is a flavone that has a yellow crystalline appearance and is usually referred to as bioflavonoid in plants. Other sources of Luteolin are thyme, chamomile tea, carrots, olive oil, rosemary leaf, green pepper and lemon. This also contributes to the yellow colour exhibited by the plant and why it is used as cosmetic on skin and nails and also the yellow-like colour seen when used as a counter stain in Gram staining technique for bacteria. The n-hexane solvent fraction of Pterocarpus soyauxii yielded six different compounds. The four compounds identified were: Malvidin 3-p-hydroxylbenzolsophoroside, Malvidin 3-acetylatedsophoroside, Malvidin and Tectoridine. Malvidin is an anthocyanin (flavonol) in the group of flavonoid (polyphenol) found abundantly in berries (bilberry and blueberry). The diversity of anthocyanins are due to the number and position of hydroxyl and methoxyl groups on the basic anthocyanidin skeleton; the number and positions at which sugars are attached, and also the extent of acylation and the identity of the acylating agent. The intensity and type of the colour of anthocynins is affected by the number of hydroxyl groups: if more methoxyl prevail, then redness increases [25].
5. Conclusions
- The dye stuff of these plants have been evaluated for the first time. These extracts have staining properties but stained poorer than the conventional synthetic stains used in the laboratory. This is because crude extracts was used for the staining procedures. It is therefore recommended that a purer form of the plants’ extracts could be used to improve their staining ability. It was also discovered that dye stain from P. soyauxii stem extract stains fungi and human appendix tissue but with little differentiation. In addition collaboration with a chemist is also very necessary.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML