-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2018; 8(1): 8-14
doi:10.5923/j.plant.20180801.02

Scarification and Soaking in Fluridone for Manipulation of Germination of Seeds from Immature Allanblackia floribunda Fruits
Cynthia Smooth, Elsie I. Hamadina
Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Rate of germination and the timing of sprouting has been significantly increased and shortened by propagating Allanblanckia seeds obtained from immature fruits rather than seeds from mature fruits. Also, soaking scarified seeds from immature fruits in 10 µM Fluridone for 6 or 12 hr has led to 72% germination in 75 days. Unfortunately, the effect of Fluridone on un-scarified seeds from immature fruits is not known and, the optimum soak duration for inducing the maximum effect on germination is not clear. Therefore, the objective of this study was to determine the effect of 0, 6, 12, 24 and 48 h of soak in 10 µM Fluridone on the timing and percentage germination of mature un-scarified and scarified Allanblanckia seeds extracted from immature fruits. Results showed that scarified seeds commenced germination after a short period (about 50 days after treatment) while germination was delayed in un-scarified seeds (about 100 days after treatment). Rate of germination in scarified seeds was higher (≥ 38% in 77 d) than un-scarified seeds (≤5% in 100 d). Soaking un-scarified seeds in 10 µM Fluridone led to only about 10% germination (plumule). Soaking scarified seeds in 10 µM Fluridone significantly (p<0.05) shortened the duration to radicle and plumule emergence and increase early seedling growth compared with the control. By testing a wider range of soak durations (i.e., durations greater and less than 12 h), it is clear that the optimum soak duration for germinating Allanblackia seeds from immature fruits is 6 h in 10 µM Fluridone (i.e., 70% radicle emergence in 65 d and 50% plumule emergence in 98 d).
Keywords: Soak Duration, Fluridone, Percentage Germination, Scarified, Un-scarified, Allanblackia floribunda
Cite this paper: Cynthia Smooth, Elsie I. Hamadina, Scarification and Soaking in Fluridone for Manipulation of Germination of Seeds from Immature Allanblackia floribunda Fruits, International Journal of Plant Research, Vol. 8 No. 1, 2018, pp. 8-14. doi: 10.5923/j.plant.20180801.02.
Article Outline
1. Introduction
- Allanblackia belongs to the family Clusiaceae. It is a dioecious tree found in the Equatorial Rain Forest of West, East and Central African regions. It is known in English as “Tallow tree” [1]. There are nine species of the genus Allanblackia, these are: A. floribunda Oliver, A. gabonensis, A. kimbiliensis, A. kisonghi, A. marienu, A. parviflora, A. stanerana, A. stuhlmannii and A. ulugurensis. All nine species of Allanblackia are native to the humid tropics of Africa [2, 3].Within the last decades, A. floribunda seeds have become a subject of international interest to many commercial enterprises because of the uniqueness of its edible fat. The fat is used in the production of healthy food products, such as margarine, which are low in trans-fat (unsaturated fats) [4]. The high value of the Allanblackia fat is the drive behind the current high demand for Allanblackia seeds. Unfortunately, the seeds are still gotten mainly from the wild and so, its supply is way below its demand [5]. Domestication of Allanblackia seed is one important way to achieve increased number of trees and hence seed number but seed germination is low due to poor seed germination rate (< 5% over 7 to 24 months) [6].The easiest way to produce large number of Allanblackia trees is by growing the seeds [7]. Unfortunately, the domestication effort by seed propagation is hindered by the lack of methods to induce spontaneous and high rate of seed germination [8, 9]. Although, the tree can be raised through vegetative propagation it requires high skill for success, the cost of production is high [10] and the trees are mostly not erect. Furthermore, the fact that seedlings are still required as root stock in vegetative propagation strengthens the need to develop method(s) for inducing precocious seed germination [7]. Dormancy is the inability of a viable seed to germinate under favourable conditions. In recalcitrant seeds like Allanblackia, the presence of hard seedcoat is a known cause of dormancy. The World Agro Forestry Centre (ICRAF) has obtained up to 40% germination over 10 months by scarifying the hard seedcoat of seeds from mature fruits and incubating (storing) them in black polythene bags [6]. Shorter duration (<45d) to germination was obtained by propagating viable seeds from immature fruits [16a]. Such seeds had only a thin seedcoat. However, the possible role of endogenous chemicals like abscisic acid (ABA) in the control of dormancy of un-scarified Allanblackia seeds have not been well studied. In many seeds that exhibit dormancy, abscisic acid concentration is found to increase as the seeds commence dormancy and decline as the seeds progress towards their natural germination time [11]. The commencement of dormancy in this case, is thought to be triggered by the onset of desiccation and hard seed coat formation; an attribute of the last phase of seed development [12]. Fluridone has been used severally to induce early germination in dormant seeds. It is a chemical that inhibits the conversion of phytoene to the plant hormone abscisic acid (ABA) [13]. Fluridone is known to encourage early germination by indirectly reducing ABA concentration in plant parts that exhibits dormancy [14, 15]. In Allanblackia, [16] has shown that Fluridone can increase percentage germination to 70-90% and shortened the duration to sprouting of scarified seeds to 3-4 months by soaking the seeds in 10 µM than 30 µM Fluridone for 6 or 12 h with 12 hr of soak being more effective than 6 hr. This finding is remarkable when compared to what happens in nature. In nature, only less than 10% germination over 24 months is obtainable from un-scarified seeds obtained from mature Allanblackia fruits. However, the effect of Fluridone on un-scarified seeds from immature Allanblackia fruits is not well documented. Moreover, since the rate of germination tends to increase with prolong soak in 10 µM Fluridone [16], this study questioned the effect of longer soak durations (<12 h in 10 µM Fluridone) on earliness and percentage germination of scarified and un-scarified Allanblackia seeds obtained from immature fruits. Any success at inducing germination over a short period in un-scarified seeds from immature fruits will reduce the drudgery associated with scarification and promote the use of un-scarified seeds from immature fruits. Thus, the objective of this study was to determine the effect of five soak durations in 10 µM Fluridone on the timing and percentage germination of un-scarified and scarified Allanblackia floribunda seeds extracted from immature fruits.
2. Materials and Methods
2.1. Experimental Area
- The study was carried out in the Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Nigeria.
2.2. Source of Seeds
- Allanblackia floribunda fruits were collected from the same tree in Mgbu Ndoki of Obigbo Local Government Area, Rivers State on the 15th of September, 2014. The fruits were carefully placed on plantain leaves for about 2 weeks to soften them for seed extraction. Allanblackia floribunda was chosen in this study because it is the most predominant species found in Nigeria. The fruits were plucked and so they were expected to be immature. Mature fruits usually detach from the abscission zone, drop and present splits or cracks on the fruit's surface.
2.3. Assessment of Fruit and Seed for Maturity
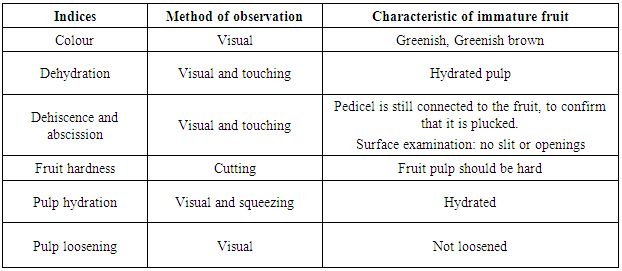
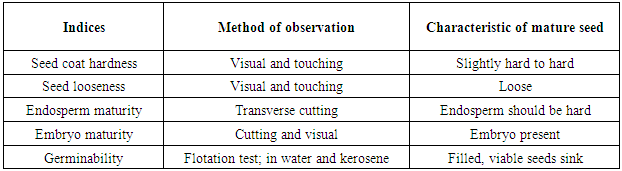
- One fruit was sampled for fruit and seed maturity assessment using indexes detailed by [17] (Tables 1 and 2).
|
|
2.4. Seed Extraction and Seed Processing
- After about two weeks of fruit softening, the seeds were extracted from the pulp of each fruit by splitting the fruits. There were two groups of seeds with unequal number of seeds. The first group of seeds were mechanically scarified without causing injury to the endosperm while all the seeds in the second group were not scarified. The seeds were tied up separately in a moistened black polythene bag and kept in a dark cupboard. This was done to prevent the seeds from drying out. One day after seed scarification, each seed was weighed to determine average seed weight.
2.5. Incubation Box and Sterilization
- Plastic containers were used to incubate the seeds in this experiment. Eighteen grooves were made on the base of each container prior to surface sterilization in 5% sodium hypochlorite. The grooves were important in preventing the seeds from rubbing themselves.
2.6. Experimental Treatment
- There were two factors in this study: Factor 1 – seed coat treatment at two levels (scarified and un-scarified), Factor 2 – soak duration in 10 µM Fluridone at 5 levels (0 h, control), 6, 12, 24 and 48 h) to give ten treatment combinations.
2.7. Seed Treatment Solutions
- Seeds were weighed and then, the pseudo seeds were identified and removed using flotation test. Flotation test was done swiftly to avoid the seeds from absorbing water. Thus, only filled, viable seeds were used in this experiment. The seeds were then surface sterilized in 0.5% sodium hypochlorite for 5 mins and rinsed twice in sterile distilled water. Then, the seeds were soaked in 10 µM Fluridone for 6, 12, 24 or 48 h. In the controls, (0 h 10 µM Fluridone scarified and un-scarified) the seeds were incubated after the sterilization and rinsing activities.
2.8. Seed Incubation
- Treated and untreated (control) seeds were incubated in transparent containers and all seeds per container received the same treatment. Thus, each container represented one of the ten treatment combinations. Each container contained eighteen seeds placed in grooves. The containers were then wrapped in black polythene bags and placed on benches in a screenhouse for 16 weeks. The containers were opened once every week for observation of germination.
 | Plate 1. Incubated seeds in a plastic container |
2.9. Micro Climatic Condition in Screenhouse
- The screenhouse was roofed with transparent plastic roofing sheets and the sides were made of transparent polyethylene sheets (essence of this was to generate heat inside the screen house). Temperature and relative humidity inside and outside the screenhouse was monitored using a 433 MHZ cable free Oregon scientific sensor model BTHR968.
2.10. Data Collection
- All seeds were observed weekly for seed rot, plumule and radicle emergence. The percentage germination, plumule and radicle length and number of leaves were taken at the end of the experiment to avoid contamination. Data collection commenced 2 weeks after seed incubation and then weekly thereafter.
2.11. Data Analysis
- The duration from treatment to plumule or radicle emergence was analysed using survival data analysis (Kaplan-Meier estimates) and run on Genstat® Discovery by VSN International Ltd, 10th edition. Survival data analysis is used for analysing data where the outcome variable is the time until the occurrence of an event of study (e.g., shoot bud appearance, rot, etc). It is even more imperative to use survival data analysis in the above type of analysis, as some seeds in the study remain alive (not germinating) longer than the period the study [18]. Thus, for such seeds, the duration to germination is unknown but the model considers it to be alive until the end of the study, and so, the survival data analysis model considers the presented duration for such seeds as censored. Censored data represents a missing data for un-germinated seeds and the value is given as one (1) while those that germinate during the study are given as zero (0). Percentage germination was calculated as number of emerged seed divided by the total number of seeds in each treatment. Seedling growth was assessed to provide preliminary indication of the effect of Fluridone on early seedling growth. Data on plumule and radical length was analysed using average and standard deviation. Number of leaves was first transformed using square root transformation and then averages and standard deviations were calculated.
3. Results and Discussion
3.1. Assessment of A. floribunda Fruit for Maturity
- Colour: the plucked fruits used in this study were greenish brown. The colour of the fruit suggested that the fruits were immature. Dehydration: The picked fruits used in this study appeared slimy and hydrated. Dehiscence and abscission: Surface examination was made on the fruit and it was observed that there were no slits, opening or cracks. Pedicels were still attached to the pod indicating that the pods were plucked from the tree. The pedicles were hard to detach from the pods at the abscission point. Hardness of fruits: This was observed by cutting through into the pods with knives, the fruits were difficult through indicating that the fruits were immature. Pulp hydration and loosening: This was done by visual and squeezing method. By squeezing it was observed that the pulp was hydrated and by visual, it was observed that the pulp was not loosened. Thus, based on the assessments above, the fruits used in this experiment were not fully mature.
3.2. Assessment of A. floribunda Seeds for Maturity
- Hardness of seed coat: The seed coat (testa) was neither hard nor soft. It was easy to remove the seed coat even without much pressure. Looseness of seeds: The seeds were difficult to extract from the pulp because, they were tightly held in the fruit pulp. Endosperm and embryo maturity: the endosperm was hard to cut through and it was slimy, suggesting that the endosperm was mature. Embryo was present, indicating that the embryo was mature.Seed Germinability: using water and kerosene test (liquid with density greater than 1), it was observed that the seeds were viable because they sunk to the bottom of the container indicating that the seeds were filled and germinable.
 | Plate 2. Presence of embryo and endosperm in mature seed from immature fruit |
3.3. Seed Moisture Content
- Seed weight (fresh weight basis) after extraction ranged from 7.0 - 9.3 g in un-scarified seeds while it ranged from 5 - 6.9 g in scarified. Average seed dry weight/seed was 6.16 g and 5.15 g in un-scarified and scarified seeds respectively. Percentage moisture content of the seeds was 7.2% and 10.2% for un-scarified and scarified seeds respectively.
3.4. Seed Germination
3.4.1. Rate of Shoot Bud Emergence
- From 1 to 6 weeks after seed treatment, no visual sign of the presence of shoot bud (plumule) emergence was observed. At 7 weeks after seed treatment, some of the seeds soaked in 10µM Fluridone (FLU) for 48 hrs began to emerge shoot bud (Plate 3). More shoot bud emergence was observed at least every two weeks with respect to time. In the control, (0 h soak in 10µM FLU), shoot bud emerged at 11 weeks (77 days) after seed treatment and the rate of germination was 38.9%. Seeds soak for 12 h in Fluridone gave a late shoot bud emergence at 14 weeks (98 days) after seed treatment but also gave the highest rate of germination at 72 % for scarified seeds. In un-scarified seeds, seeds soaked for 6 h in 10 µM Fluridone and those soaked for 24 h in 10 µM Fluridone produced 11.1% and 5.6% shoot buds emergence at 112 and 105 days (4 months) after seed treatment respectively.
 | Plate 3. Shoot bud emergence |
3.4.2. Rate of Root bud Emergence
- Root bud emergence (radicle germination) was first noticed at 4 weeks after treatment (4 WAT), and this was observed on scarified seeds that were soaked for 24 h soak in 10 µM Fluridone (Plate 4). By 7-11 weeks after treatment, more root bud emergence was observed in all of the treatment levels in the scarified seeds.
 | Plate 4. Root bud emergence |
3.4.3. The Effect of Seed Coat Treatment on the Duration from Soak in 10 µM Fluridone to Germination
- Result from survival data analysis shows that the duration from treatment to germination (plumule emergence) was significantly (P<0.001) shorter in scarified seeds than that of non-scarified seeds (Fig. 1). Scarified seeds survived (did not germination or remained dormant) for only a short period (less than 50 d), while non-scarified seeds survived (remained dormant) for very long (greater than 100 d). Also, the log-cumulative hazard curve over time showed that each scarified seed has a high tendency to germinate before the end of the study than non-scarified seeds. Only less than 10% of seeds germinated among the non-scarified seeds.
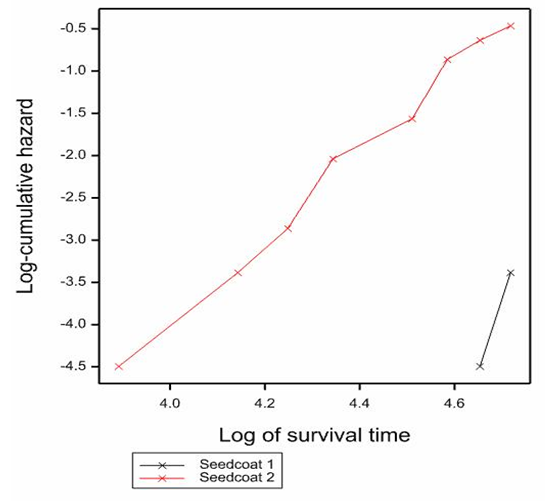 | Figure 1. Effect of seed coat treatment on duration from treatment in 10 µM Fluridone to germination in scarified (seedcoat 2) and non-scarified (seedcoat 1). Log-rank statistics 46.7 at p<0.001 |
3.4.4. Effects of Soak Duration in 10 µM Fluridone on the Duration from Treatment to Radicle Emergence
- Because the rate of germination among non-scarified seeds was very low, this analysis was conducted using germination data from only scarified seeds. The result showed that soaking seeds in 10 µM Fluridone led to significantly (p<0.01) earlier radicle emergence than the control (Figure 2). Up to 50 % but less than 75 % germination occurred in 55 days and 65 days by soaking for 12 h and 6 h respectively. By soaking the seeds for 24 h in Fluridone, less than 50% radicle emergency occurred at 75 days. By not soaking the seeds in Fluridone (Control), and by soaking the seeds for 48 h in Fluridone, 25% radicle emergence occurred after 100 days and more.
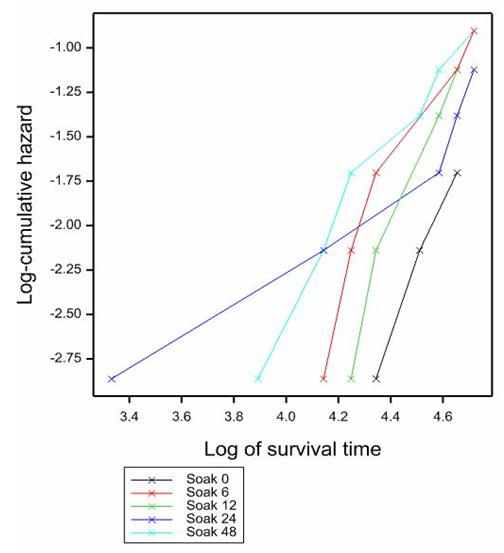 | Figure 2. Effect of different soak durations in 10 um Fluridone on radicle emergence of scarified seeds. Log-rank statistics 1.7 at P<0.05 |
3.4.5. Effect of Soak Duration in 10 µM Fluridone on the Duration from Treatment to Plumule Emergence
- The result showed that soaking seeds in 10 µM Fluridone led to significantly (P<0.05) earlier plumule emergence than the control (Fig. 3) which led to 25% plumule emergence in 105 days. Up to 25% germination occurred in 91 days by soaking for 48 h and 6 h. By soaking for 24 h, plumule emergence was 25% in 98 days, and by soaking for 12 and 6 h plumule emergence was attained at 25% and 50% in 98 days. However, for un-scarified seeds there was no data analysis because data from seeds soaked in control (0 h), 12 h and 48 h were all censored data (no germination) except for 6 h and 24 h which gave more than 10 % and 5% germination respectively.
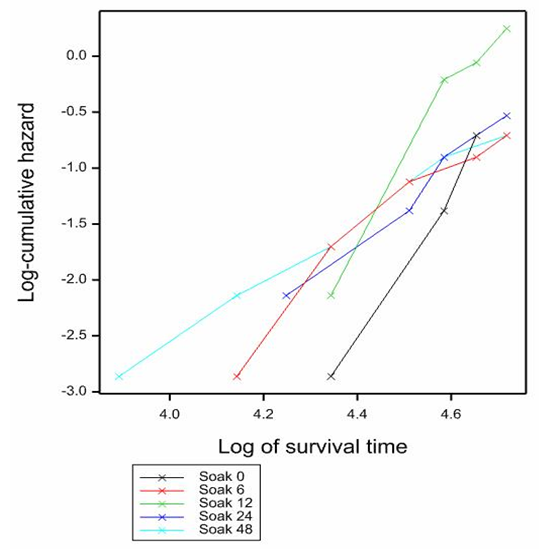 | Figure 3. Effect of different soak durations in 10 µM Fluridone on plumule emergence of scarified seeds. Log-rank statistics 4.46 at P<0.05 |
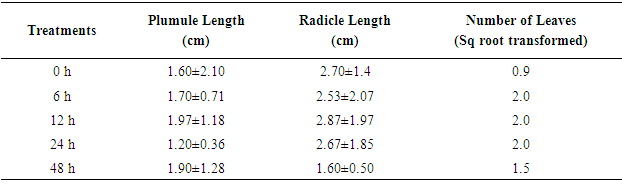
3.5. Effect of Fluridone on Seedling Growth
- Seedling growth was evaluated at the end of the study (119 days after treatment). The results showed that the highest number of leaves was obtained by soaking seeds in 6, 12 and 24 h in Fluridone (Table 3). In the control (0 h), the number of leaves was fewer than that in 48h but two times fewer than those in 6, 12 and 24 h in Fluridone treatments. For plumule and radical length, 12 h soak in Fluridone gave the highest length of 1.97±1.18 cm and 2.87±1.97 cm respectively. In the control plumule and radicle length was 1.6±2.1 cm and 2.7±1.4 cm respectively. This result suggests that seedling growth is not negatively affected by Fluridone.
|
4. Conclusions
- This study has unequivocally shown that scarification is required, to enhance germination, when seeds with slightly hard seedcoat are collected from immature fruits. Hence, the collection of seeds from greenish fruits (immature fruits) isn’t the major criteria for enhancing germination but the age of the seeds. Seeds with slightly hard seedcoat are low in moisture content and more difficult to germinate. This study has also shown that fluridone is more effective at inducing seed germination in scarified than un-scarified seeds. Hence, techniques (other than scarification) that can induce high rates of germination in un-scarified seeds must be sought for. Future studies may consider the use of technics that improve the permeability of the seedcoat of Allanblackia seeds to gases and solutions. Since pods of Allanblackia are still collected from the wild, effort should be made to domesticate the plant in order to meet the high demand for the oil rich seed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML