Carolina Chaves Ramos1, Adriana Lima de Sousa2, Cibele Maria Stivanin de Almeida1, Rodrigo Rodrigues de Oliveira1
1Laboratório de Ciências Químicas, Universidade Estadual do Norte Fluminense Darcy Ribeiro-UENF, Campos dos Goytacazes, RJ, Brazil
2Instituto Federal Fluminense Campus Campos Guarus, Campos dos Goytacazes, RJ, Brazil
Correspondence to: Rodrigo Rodrigues de Oliveira, Laboratório de Ciências Químicas, Universidade Estadual do Norte Fluminense Darcy Ribeiro-UENF, Campos dos Goytacazes, RJ, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Solanum L. is a large, diverse and important Solanaceae genus, which taxonomy and infrageneric relationships are still unresolved, as well as its evolutionary history. Biosynthesized from the mixed pathway (acetate/shikimate) and considered good systematic markers, flavonoids are widely distributed among plant species, as also in Solanum. Through analysis of flavonoid chemical data, this work aims to study evolutionary polarizations and contribute towards the comprehension of species phylogenetic relationships in the genus. The chemosystematic analysis was conducted through calculation of flavonoids chemical parameters and employing multivariate statistical analysis (factor analysis). Chemical data survey led to the identification of 479 metabolites from the mixed pathway. The results obtained demonstrate Solanum species prefer to biosynthesize hydroxylflavonoids. However, when oxy-groups are protected, choose O-glycosylflavonoids rather than O-methylflavonoids. The chemometric analysis showed derivation of eight Leptostemonum species, clearly separated from the rest of Solanum due to O-methylation. This feature suggests a possible advancement of Leptostemonum species. At last, considering this preliminary analysis, it was possible to identify remarkable trends in flavonoid evolution in Solanum species.
Keywords:
Solanum, Solanaceae, Chemosystematic, Mixed Pathway, Flavonoids
Cite this paper: Carolina Chaves Ramos, Adriana Lima de Sousa, Cibele Maria Stivanin de Almeida, Rodrigo Rodrigues de Oliveira, Solanum Chemosystematic Aspects: Analysis of Mixed Pathway (Acetate/Shikimate) Special Metabolites, International Journal of Plant Research, Vol. 7 No. 3, 2017, pp. 75-82. doi: 10.5923/j.plant.20170703.03.
1. Introduction
Solanum L. is the largest Solanaceae genus and comprises about 1,400 species with worldwide distribution [1, 2]. Its species possess great social and economic value as food, like potato (S. tuberosum) and tomato (S. lycopersicum), as also as medicine, such as jurubeba (S. paniculatum).Over time, Solanum species have been studied and classified by many taxonomists based on morphological and anatomical characteristics. Dunal (1813; 1852), D’Arcy (1972; 1991), Nee (1999) and Hunziker (2001) published the main Solanum works [3-8], being D’Arcy’s classification system (1972) the most used and accepted worldwide.The advent of molecular phylogeny contributed towards a better understanding of Solanum taxonomy. From the 90’s, a series of studies conducted using this approach [9-12] resulted in an infrageneric classification of Solanum in thirteen subspecies group: African non-spiny, Archaesolanum, Brevantherum, Cyphomandra, Dulcamaroid, Geminata, Leptostemonum, Morelloid, Normania, Potato, Regmandra, Thelopodium and Wendlandii-Allophyllum [12]. While Solanum genus presents great morphological diversity, its species exhibit uniform aspects, like pentamerous perianth and androceus, connivent stamens and poricidal anther dehiscence. Solanum also has species showing different habit types, from herbs to small trees, shrubs and vines [13, 14]. Due to the size and morphological diversity found in the genus, problems concerning taxonomical classification are not well comprehended yet.Chemical evolution displayed in Solanum results from an evolutionary channeling manifested by gradients related to morphology and metabolism. Although recently plant systematics has elucidated Solanum taxonomy, many controversies are still unresolved [9-12].In this study, special metabolites from the mixed pathway were used as systematic markers to evaluate Solanum genus diversification. Thus, these chemical data can be exploited based on the assumption flavonoids are good systematic markers, since its biosynthetic relationships and evolutionary scheme are well known [15], allowing to distinguish primitive metabolites from more advanced ones in angiosperms.This work aims to study Solanum evolutionary polarizations through analysis of its chemomarkers occurrence pattern. It is intended to help in a better understanding of species phylogenetic relationships in the genus, employing chemosystematic methodology approach.
2. Materials and Methods
2.1. Chemosystematic Methodology
Chemical data research was performed on Scifinder database covering papers published from 1902 to 2016. Survey was conducted using as keywords the name of species belonging to each Solanum subgenus, following Weese and Bohs (2007) phylogenetic classification [12].Metabolites biosynthesized by the mixed pathway were classified according to their flavonoid class, as well as were counted the occurrence number (ON).Chemosystematics analysis was conducted as described by Gottlieb et al (1996) methodology [16]. This approach is based on the calculation of chemical parameters for each metabolite, such as: evolutionary advancement oxidation (EAO) and skeleton specialization index (EAE); flavonoid protection and unprotection indices: EAG (O-glycosylation), EAM (O-methylation), EATP (O-total protection) and EATU (O-total unprotection).While, oxidation (O) and skeleton specialization (E) indices were determined based on the following equations 1 and 2: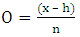 | (1) |
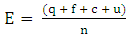 | (2) |
Where:x = number of C – heteroatom bonds;h = number of C – H bonds;n = number of carbon atoms in the molecular skeleton;q = number of broken C – C bonds in relation to a precursor;f = number of formed C – C bonds in relation to a same precursor;c = number of cyclic systems formed involving heteroatoms in relation to a same precursor;u = number of carbonic additional units in relation to a precursor;Evolutionary advancement oxidation (EAO) and skeleton specialization (EAE) indices were obtained by the following equations 3 and 4, respectively: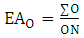 | (3) |
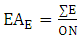 | (4) |
The phenolic substitution pattern in flavonoids was also investigated by means of evolutionary advancement indices, such as EAG (O-glycosylation), EAM (O-methylation), EATP (O-total protection) and EATU (O-total unprotection), as shown in equations 5, 6, 7 and 8: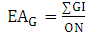 | (5) |
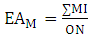 | (6) |
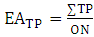 | (7) |
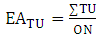 | (8) |
Where:ON: occurrence number;GI: O-glycosyl group number/ total oxy-group number;MI: O-methyl group number/ total oxy-group number;TP: oxy-group protected number/ total oxy-group number;TU: oxy-group unprotected number/ total oxy-group number;The relation between flavones (fo) and flavonols (fl) bioproduction was calculated for each Solanum subgenus [17]. This parameter consists in an important factor to establish morphological and chemical diversity relationship: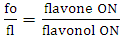 | (9) |
2.2. Multivariate Analysis
Multivariate statistical analysis was performed using factor analysis technique. Software used for data treatment was Statistica® 12 for Windows.
3. Results and Discussion
3.1. Solanum Chemical Profile
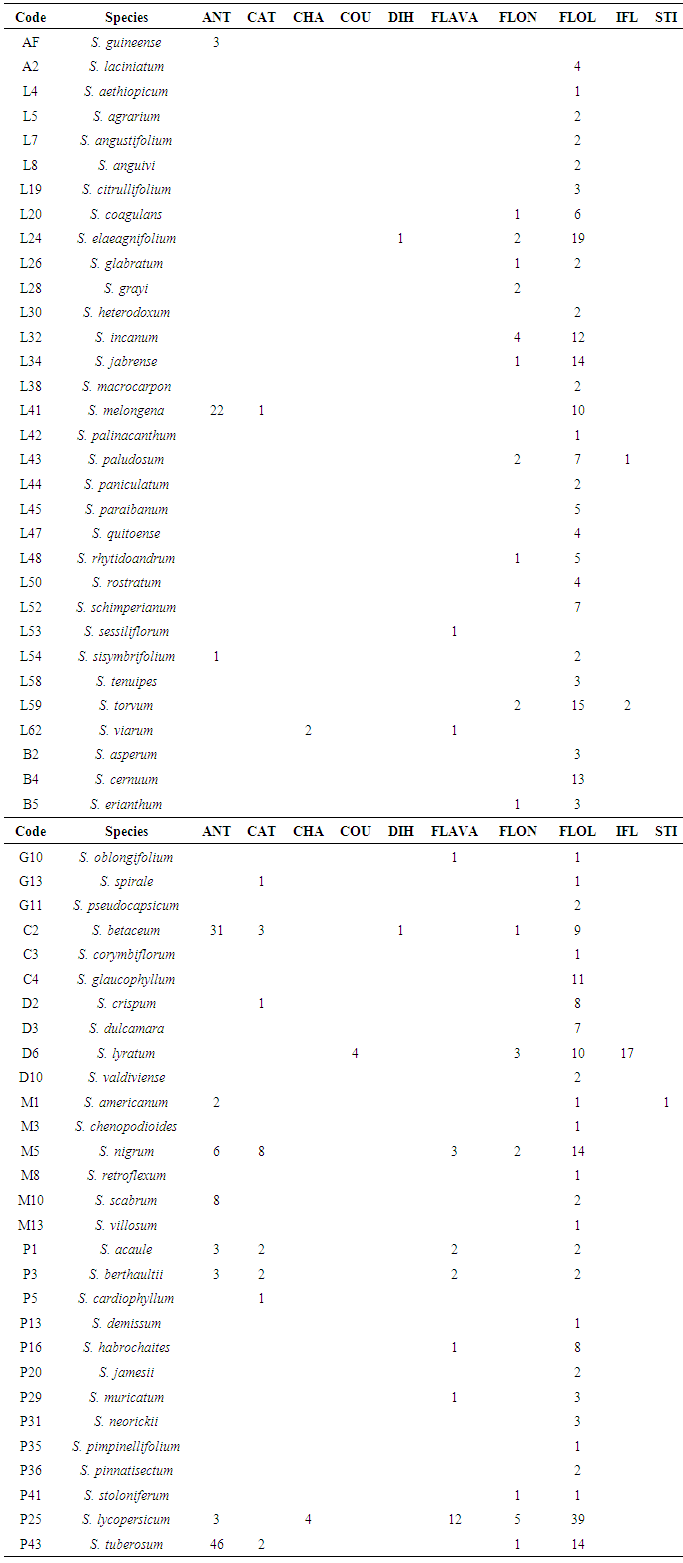
Bibliographic survey of Solanum chemical data led to 923 papers. 479 metabolites from the mixed pathway were identified. The distribution in nine Solanum subgenera resulted in the following occurrence number: Leptostemonum (ON=180); Brevantherum (ON=20); Geminata (ON=7); Cyphomandra (ON=57); Morelloid (ON=50); Dulcamaroid (ON=52); Potato (ON=106); Archaesolanum (ON=4) and African non-spiny (ON=3). Regmandra, Normania, Thelopodium and Wendlandii-Allophyllum subgenera do not have literature report of mixed pathway metabolites. Among Solanum major metabolites, it can be highlighted the predominant flavonol occurrence in nearly all subgenera, as in Leptostemonum (ON=132), Potato (ON=64), Dulcamaroid (ON=27), Morelloid (ON=20), Brevantherum (ON=19), Geminata (ON=5) e Archaesolanum (ON=4). Species belonging to Cyphomandra presents flavonol (ON=21), but anthocyanidin predominates (ON=31). No flavonol reports were found in African non-spiny subgenus, showing only anthocyanidin presence (ON=3).Regarding structural diversity of Solanum metabolites from the mixed pathway, it is noted the presence of few relevant flavonoidic classes, such as anthocyanidins (ON=82), catechins (ON=19), chalcones (ON=6), coumestans (ON=4), dihydroflavonols (ON=2), flavones (ON=29), flavonols (ON=292), flavanones (ON=24), isoflavonoids (ON=20) and stilbenes (ON=1). Table 1 summarizes the occurrence number of flavonoidic classes of each species. This feature demonstrates flavonoids bioproduction is still in transition in Solanum genus.Table 1. Occurrence number (ON) of mixed pathway metabolites of Solanum species classified according to micromolecular category; Codes: ANT (anthocyanidin), CAT (catechin), CHA (chalcone), COU (coumestan), DIH (dihydroflavonol), FLAVA (flavanone), FLON (flavone), FLOL (flavonol), IFL (isoflavonoid), STI (stilbene). Data collected on SciFinder database covering papers from 1902 to 2016
 |
| |
|
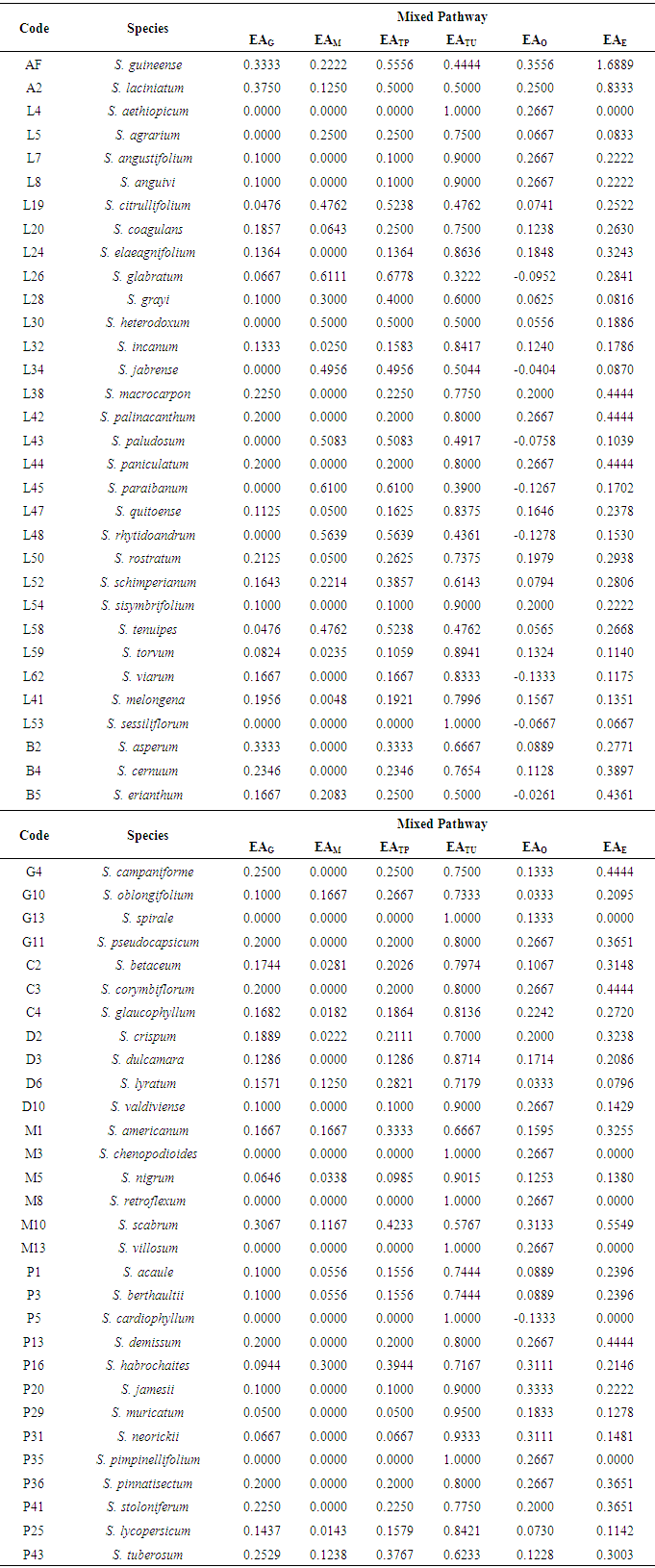
However, due to the mixed pathway homology, chemical similarity between species belonging to the same subgenus is supported by flavonoid structural variation and even by analogous evolutionary aspects [18].The study reveals that flavonoidic structural diversification occurs simultaneously and is in an intermediary phase in the genus. Solanum flavone/flavonol ratio (0.07) shows flavonols represents the most important mixed pathway subclass in all Solanum subgenera analyzed for this parameter. Flavone/flavonol ratio of Solanum subgenera are, as follows: Leptostemonum (0.12); Dulcamaroid (0.11); Morelloid (0.10); Potato (0.09); Brevantherum (0.05); Cyphomandra (0.05); Archaesolanum (0); Geminata (0).Higher prevalence of flavonols instead of flavones is a significant correlation parameter between morphological and chemical features. This aspect demonstrates a primitive character in the flavonoid chemistry of Solanum.Primitiveness is confirmed by two parameters obtained for the Solanaceae family: Sporne (IS=68) and Herbaceousness (IH=50) indices [15, 17, 19]. Solanum shows a preference in flavonol bioproduction that may indicate a more primitive taxon positioning [20], according to Bate-Smith (1962).Concerning phenolic protection and analyzing chemical modulatory indices, as detailed in table 2, it is clearly noted the trend for unprotection of flavonoidic hydroxyls in Solanum species.However, it is observed a derivation of some species belonging to the Leptostemonum subgenus from the rest of Solanum. This behavior is noted in eight Leptostemonum species (L19, L26, L30, L34, L43, L45, L48, L58), which exhibits more than 50% of O-methylation in flavonoids. When protected, others species shows O-glycosylation protection preference and in some cases, associates both O-glycosylation and O-methylation protection.Solanum flavonoidic chemistry is dominated by unprotected flavonoids. Although being a basal feature, this analysis reveals that the genus is in transition due to the slight presence of different oxygenated groups protection patterns. However, it is worth noting O-glycosylation and O-methylation are protection mechanisms elaborated by Solanum species and represent final stages in flavonoid biosynthesis [21].Thus, hydroxylflavonoids bioproduction and the absence of flavones could indicate group primitiveness [15, 20]. However, micromolecules biosynthesis deriving from acid acetic pathway shows to predominate in Solanum [22].Regarding evolutionary advancement skeleton specialization index (EAE) it is verified species have low values that do not differentiate each other. Only AF (EAE=1.6889) is far from the rest of Solanum, due to the presence of acylated and glycosylated anthocyanidin in this taxon. Solanum species presents extremely low EAO index values, which is in agreement with flavonoids oxygenation patterns found in the genus. Its flavonols demonstrates ring B [(3’,4’), (3’,4’,5’) and (5,6)] oxidation patterns, besides [(5,7) and (5,7,8)] in ring A. Thus, Solanum shows a poor oxidative evolutionary advancement, which is characteristic of basal angiosperms, such as high index of hydroxylflavonoids.EAO index and phenolic protection correlation seems to indicate evolutionary biosynthetic trends of species based upon gradual replacement of flavonoids less oxygenated and glycosylated to more oxygenated metabolites and methylated.
3.2. Chemosystematic Data: Chemometric Analysis
Factor analysis of chemosystematic parameters of Solanum species assigned to Factor 1 were O-methylation (EAM), O-Total Protection (EATP) and Total Unprotection (EATU). To Factor 2 were attributed O-glycosylation (EAG) and EAE variables. Thus, bidimensional diagram (Figure 1) represents factor analysis of 62 Solanum species, disclosing a great dispersal between them. Analysis shows Factor 1 variables causes a high species dispersal and separate them in four groups.First group is represented by eight Leptostemonum species L19, L26, L30, L34, L43, L45, L48, L58 clearly separated from the others. These Solanum species exhibit more than a half of their flavonoids oxy-groups highly protected. Besides, hydroxyls are protected exclusively by O-methylation mechanisms, as can be seen in data table (Table 1).Next, second group comprises A2, M10, B2, B5, P43, M1, P16, L52, L28, L5, G10, D6 species, characterized by double protection mechanisms. All species of this group have flavonoids protected simultaneously by O-methylation and O-glycosylation. Indices related to each species are summarized in table 2.Whereas, species B4, C2, C3, C4, D2, D3, D10, G4, G11, G13, L4, L7, L8, L20, L24, L32, L38, L41, L42, L44, L47, L50, L53, L54, L59, L62, M3, M5, M8, M13, P1, P3, P5, P13, P20, P25, P29, P31, P35, P36, P41 are included in the third group. This species set presents mostly unprotected flavonoids. When protected, it shows preference by O-glycosylation, as can be observed in table 2.Table 2. Evolutionary advancement parameters of mixed pathway metabolites of Solanum species; codes are: AF (African non-spiny), A (Archaesolanum), L (Leptostemonum), B (Brevantherum), G (Geminata), C (Cyphomandra), D (Dulcamaroid), M (Morelloid), P (Potato)
 |
| |
|
AF is distant from other Solanum species, forming a separated group due to parameters of Factor 2 (EAG and EAE).Evolutionary advancement specialization index (AF EAE: 1.6889) is determinant, being the highest of all Solanum species, due to acylated and glycosylated anthocyanidin presence in this subgenus. As can be seen in figure 1, factor 1 contributed to differentiate groups 1, 2 and 3 according to the phenolic/oxy-groups protection parameters (AEOMet, AETP, AETU). While factor 2 is important in separation of the group represented by AF from other Solanum species, due to AEOGly parameter and the advancement skeleton specialization index (EAE). Contributions of each variable to factors 1 and 2 are detailed in table 3.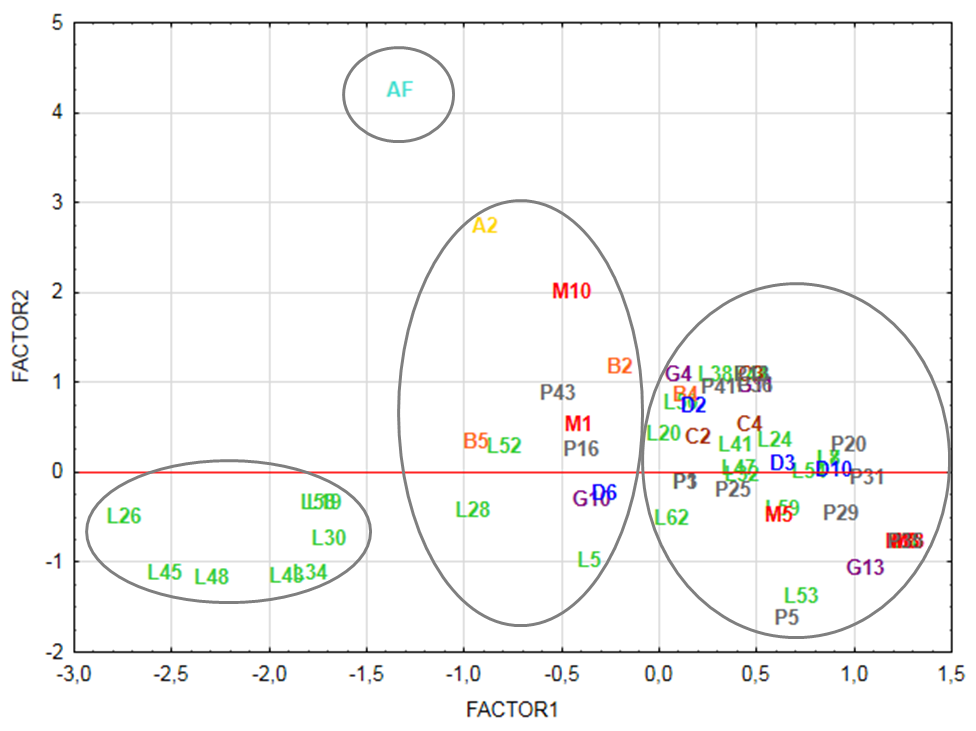 | Figure 1. Bidimensional diagram representing factor analysis of parameters of mixed pathway metabolites of 62 Solanum species; Factor 1 (EAM, EATP and EATU) and Factor 2 (EAG and EAE) |
Table 3. Variables contributions to factor 1 and 2 in factor analysis of Solanum species
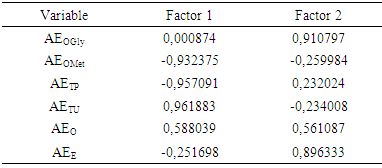 |
| |
|
4. Conclusions
Preliminary chemosystematic analysis of Solanum species allowed an estimate of evolutionary features in the genus. Moreover, it evidences micromolecular similarity in genus systematic and evolutionary context, related to structural chemical variation. Although it cannot be actually confirmed which subgenus is more primitive and which is more advanced, it is conclusive that the eight Leptostemonum species (L19, L26, L30, L34, L43, L45, L48, L58) have distinct features from other species belonging to this taxon, due to O-methylflavonoids. These species elaborate more specialized protection mechanisms by O-methylation, what could represent a transition group or even a more evolved one. An associated analysis with molecular phylogenetic and morphological characters could confirm this assumption indicated by the chemical data.Finally, it is expected that in near future, chemosystematics analysis of metabolites from the acetic acid pathway, which predominates in Solanum metabolism, could help to elucidate the evolutionary history still unresolved in this taxon.
ACKNOWLEDGEMENTS
The authors wish to thank UENF and CAPES for financial support.
References
| [1] | Frodin, D. G. 2004. History and concepts of big plant genera. Taxon, v. 53, n. 3, p. 753-776. https://doi.org/10.2307/4135449. |
| [2] | Olmstead, R. G.; Bohs, L. 2007. A summary of molecular systematic research in Solanaceae: 1982-2006. Acta Horticulturae, p. 255-268. https://doi.org/10.17660/ActaHortic.2007.745.11. |
| [3] | Dunal, M. F. 1813. Histoire naturelle, médicale et économique des Solanum. 257p. |
| [4] | Dunal, M. F. 1852. Prodromus Systematis Naturalis Regni Vegetabilis. Candolle. |
| [5] | D’arcy, W. G. 1972. Solanaceae Studies II: Typification of Subdivisions of Solanum. Annals of the Missouri Botanical Garden, v. 59, n. 2, p. 262-278. http://doi.org/10.2307/2394758. |
| [6] | D’arcy, W. G. 1991. The Solanaceae since 1976, with review of its biogeography. In: Hawkes, J. G.; Lester, R. N.; Nee, M. Solanaceae III: Taxonomy, chemistry, evolution. Royal Botanical Garden & Linnean Society, p. 75-137. |
| [7] | Nee, M. 1999. Synopsis of Solanum in the New World. In: Nee, M. et al. Solanaceae IV: advances in biology and utilization. Kew: The Royal Botanic Gardens. |
| [8] | Hunziker, A. T. 2001 Genera Solanacearum: the genera of Solanaceae illustrated, arranged according to a new system. Ruggell, Liechtenstein: Gantner Verlag xvi, 500p. |
| [9] | Bohs, L.; Olmstead, R. G. 1997. Phylogenetic relationships in Solanum (Solanaceae) based on ndhf sequences. Systematic Botany, v. 22, n. 1, p. 5-17. https://doi.org/10.2307/2419674. |
| [10] | Bohs, L.; Olmstead, R. G. 1999. Solanum phylogeny inferred from chloroplast DNA sequence data. In: Nee, M. et al. Solanaceae IV: advances in biology and utilization. Royal Botanical Gardens, p. 97-110. |
| [11] | Bohs, L. 2005. Major clades in Solanum based on ndhF sequence data. A Festschrift for William G. D'Arcy: the legacy of a taxonomist. Monographs in systematic botany from the Missouri Botanical Garden. p. 27-49. |
| [12] | Weese, T. L.; Bohs, L. 2007. A three-gene phylogeny of the genus Solanum (Solanaceae). Systematic Botany, v. 32, n. 2, p. 445-463. https://doi.org/10.1600/036364407781179671. |
| [13] | Roe, K. E. 1972. A revision of Solanum section Brevantherum (Solanaceae). Brittonia, v. 24, n. 3, p. 239-278. https://doi.org/10.2307/2805665. |
| [14] | Agra, M. F.; Nurit-Silva, K.; Berger, L. R. 2009. Flora da Paraíba, Brasil: Solanum L. (Solanaceae). Acta Botanica Brasilica, v. 23, n. 3, p. 826-842. https://doi.org/10.1590/S0102-33062009000300024. |
| [15] | Harborne J.B. 1977. Flavonoids and the evolution of the Angiosperms. Biochemical Systematics and Ecology, v. 5, p. 7-22. https://doi.org/10.1016/0305-1978(77)90013-8. |
| [16] | Gottlieb, O. R.; Kaplan, M. A. C.; Borin, M. R. M. B. 1996. Biodiversidade: um enfoque químico-biológico. Editora da UFRJ. |
| [17] | Soares, G. L. G.; Kaplan, M. A. C. 2001. Analysis of flavone-flavonol ratio in Dicotyledoneae. Botanical Journal of the Linnean Society, v. 135, p. 61-66. https://doi.org/10.1111/j.1095-8339.2001.tb02369.x. |
| [18] | Gottlieb, O. R. 1982. Micromolecular evolution, systematics and ecology: an essay into a novel botanical discipline. Springer-Verlag. https://doi.org/10.1007/978-3-642-68641-2. |
| [19] | Sporne, K. R. 1980. A re‐investigation of character correlations among dicotyledons. New Phytologist, v. 85, n. 3, p. 419-449. http://dx.doi.org/10.1111/j.1469-8137.1980.tb03180.x. |
| [20] | Bate-Smith, E. C. 1962. The phenolic constituents of plants and their taxonomic significance I. Dicotyledons. Botanical Journal of the Linnean Society, v. 58, p. 95-173. https://doi.org/10.1111/j.1095-8339.1962.tb00890.x. |
| [21] | Soares, G.L.G. 1996. Polarizações da química flavonoídica em linhagens vegetais. Rio de Janeiro. Tese de Doutorado. NPPN. UFRJ. 133p. |
| [22] | Ramos, C. C.; Sousa, A. L.; Almeida, C. M. S.; Oliveira, R. R. Unpublished data. |



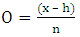
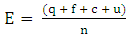
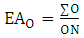
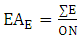
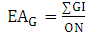
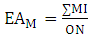
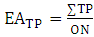
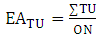
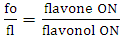

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

