-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2017; 7(3): 65-74
doi:10.5923/j.plant.20170703.02

Performance of Sorghum Stay-green Introgression Lines Under Post-flowering Drought
Nasrein Mohamed Kamal1, 2, Yasir Serag Alnor Gorafi2, 3, Abdelbagi Mukhtar Ali Ghanim1, 4
1Biotechnology and Biosafety Research Center, Agricultural Research Corporation, Shambat, Khartoum North, Sudan
2Arid Land Research Center, Hamasaka, Tottori, Japan
3Agricultural Research Corporation, Wad Medani, Sudan
4Plant Breeding and Genetics Laboratory, FAO/IAEA Joint Division, International Atomic Energy Agency (IAEA), Seibersdorf, Austria
Correspondence to: Abdelbagi Mukhtar Ali Ghanim, Biotechnology and Biosafety Research Center, Agricultural Research Corporation, Shambat, Khartoum North, Sudan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Stay-green sorghum exhibits greener leaves and stems during the grain filling period under drought conditions, resulting in increased grain/mass yield, and lodging resistance. To improve sorghum grain yield (GY) under post-flowering drought, we developed 46 BC2F4 stay-green introgression lines (BILs) from a cross between ‘Tabat’ × B35 by marker-assisted backcrossing (MAB). These BILs had one or more of the four stable stay-green QTLs (Stg1 to Stg4) from the donor B35. We evaluated these lines to examine the progress made in transferring the drought tolerance under irrigated and rain-fed environments in Sudan. The introgression of the stay-green QTLs enhanced post-flowering drought tolerance and increased the GY and biomass of ‘Tabat’. Under drought conditions, some BILs had GY and biomass higher than ‘Tabat’. By contrast, under irrigation, the GY of the BILs was lower than that of ‘Tabat’ indicating that further backcrossing is necessary to restore ‘Tabat’ yield potential. Stg1 was the best QTL in term of GY. QTL pyramiding increased the tolerance, however, it might not always be necessary. Based on the biplot analysis; several genotypes will be selected and advanced to further backcrossing. The study provided evidence that MAB with stay-green QTLs can enhance sorghum yield under post-flowering drought in Sudan and similar agro-ecological zones.
Keywords: Sorghum, Stay-green, Post-flowering drought, Marker-assisted selection
Cite this paper: Nasrein Mohamed Kamal, Yasir Serag Alnor Gorafi, Abdelbagi Mukhtar Ali Ghanim, Performance of Sorghum Stay-green Introgression Lines Under Post-flowering Drought, International Journal of Plant Research, Vol. 7 No. 3, 2017, pp. 65-74. doi: 10.5923/j.plant.20170703.02.
Article Outline
1. Introduction
- Sorghum (Sorghum bicolor (L.) Moench) is unique among the major cereals in that its grain is the staple food of the world’s poorest people, who have the lowest food security and who live primarily in the semiarid tropics. Drought stress is a major constraint to crop production in semi-arid tropics [1]. In Sudan, more than 75% of sorghum is grown in rain-fed cultivations [2], and water scarcity is the major limiting factor because periods of drought can occur at any stage of crop development. Therefore, improving drought tolerance of sorghum has been a challenge for plant breeders. The response of sorghum to drought stress depends on the growth stage at which the stress occurs [3, 4]. The response to post-flowering drought is evident when moisture stress occurs during the grain development stages. Drought stress during grain filling results in rapid premature plant senescence [3]. Genotypes that can tolerate post-flowering drought stress maintain active photosynthesis when subjected to water stress during the grain-filling period. Such genotypes are described as possessing the “stay-green” trait [4].Molecular markers, associated with stay-green, were identified and characterized in some accessions, such as B35, E36-1, and SC56. The putative QTLs (Stg1 to Stg4) for the stay-green trait from B35 were identified based on four mapping populations [5-10]. Examination of the stay-green QTL profiles of the best and poorest stay-green lines indicated that three stay green QTLs, Stg1, Stg2 and Stg3, appear to be important for the expression of this trait when the percent phenotypic variation and the consistency in different backgrounds and different environments is considered [7]. Walulu et al. [11] found that, in sorghum, the stay-green trait is controlled by a major gene that expresses different levels of dominant gene action, depending on the environment. Maintenance of a greater green leaf area during the latter half of grain filling is related to a greater grain yield under post-flowering moisture deficits [12]. The relationship between stay-green and grain yield varies in response to both the environment and the genetic background. The transfer of the stay-green trait into elite lines is expected to be broadly beneficial for increasing yield in a wide range of environments [13]. Reddy et al. [14] validated the importance of stay-green QTLs and detected new QTLs influencing the stay-green related traits. These authors found that Stg2, Stg3 and StgB were consistently prominent in their expression. Vadez et al. [15] and recently Borrell et al. [16, 17] studied the effect of the stay-green QTLs 1-4 on plant performance under post-flowering drought comprehensively. They concluded that the stay-green QTLs impacts sorghum performance positively under post-flowering drought through modification of canopy development, leaf anatomy, root growth, and water uptake. However, this positive impact depends on the environment and the interaction between the stay-green QTLs and the genetic background. The stay-green QTLs were ranked based on their contribution to the stay-green phenotype as Stg2, Stg1, Stg3, and Stg4 in their order of merit [18]. All these reports indicate unequivocally the potential of the stay-green trait in developing drought tolerant sorghum, but they indicate that the magnitude of the contribution of the stay-green QTLs to the yield of sorghum under post-flowering drought depends largely on the genetic background and the environments. In addition, only few reports discussed the effect of multiple stay-green QTLs on plant yield under post-flowering drought. This paper aimed at evaluating the performance of an early back cross generation of stay-green introgression lines carrying single and multiple stay-green QTLs in four different environments in Sudan. In these environments and the similar agro-ecological zones in sub-Saharan Africa stay-green effect on enhancing sorghum grain yield has not been explored adequately to the best of our knowledge.
2. Materials and Methods
2.1. Plant Materials
- We crossed the drought sensitive, high yielding, farmers-preferred cultivar ‘Tabat’ [19] as a recurrent parent to the stay-green donor B35 for two generations of backcrossing (BC2F1) [20]. ‘Tabat’ is a white grained high yielding cultivar grown under irrigation in Sudan. B35, a derivative of an Ethiopian durra and Nigerian landrace, has low yield but it is the source of stay-green that has been used in most of the genetic studies and associated programs related to terminal drought [21]. The F1, BC1F1 and BC2F1, were genotyped to identify and further cross individuals carrying the stay-green loci of B35 (Stg1, Stg2, Stg3 and Stg4) (Supplementary Fig. 1, Supplementary Table 1). Forty-six BC2F4 plants with one, two or three stay-green QTLs in different combinations were produced and evaluated for terminal drought tolerance under irrigated and rain-fed conditions.
2.2. Irrigated Experiment
- An irrigated experiment was conducted at Alfaki Hashim Research Farm, Khartoum North (15.841923° N, 32.552671° E) with vertisols. We imposed two water regimes: 1) recommended optimum irrigation every 10 to 14 days until harvest; 2) cessation of irrigation at 50% flowering to impose post-flowering drought. When irrigation ceased, the plants depended on stored soil moisture and thus experienced prolonged and severe post-flowering (terminal) drought stress during grain filling. This practice enables the evaluation of terminal drought tolerance due to the stay-green trait [12, 21]. The experiment had three replicates. Plot size was three rows of five-meter-long with 0.8 m inter-rows spacing in a 6 (blocks per replicate) × 8 (genotypes per block) unbalanced lattice design. Five random plants from the inner row were tagged in each plot at flowering for data collection.
2.3. Rain-Fed Experiments
- These experiments were conducted at two locations with different rainfall levels. The one with relatively high rainfall (Optimum) at South Gedaref site (14°34'N, 35°54'E) has an average annual rainfall of 514 mm, most of which occurs between July and September. The soil clay content is very high and generally 75% to 80%. The color of the soils is very dark grayish brown. The soils are moderately fertile, the organic matter, nitrogen and potassium contents of the soil are low, but there is no deficiency of other plant nutrients. The water holding capacity of the soil material is very high and allows crops to grow on stored water during dry spells and long after the rainy season.Low rainfall characterizes El Obeid site (13°11′N, 30°13′E), where the average annual rainfall is 271 mm (falling between July and September). The soil is sandy clay with 25% clay, 67% sand, 8% silt and 0.7% organic matter. As in the irrigated experiment, we used the same experimental design, plot size and sample size of five plants from each plot for data collection.
2.4. Data Collection
- Stay-green (delayed leaf senescence) was scored by measuring the percent of greenness (%G) at both grain filling (GF) and maturity (M). %G is green leaves as a percentage of the total number of leaves. We harvested five plants from the middle row of each plot to determine the final grain yield (GY) per plant and the fresh plant biomass (PB). Yields were expressed as the total weight of grains of each plant.
2.5. Statistical Analysis
- The irrigated experiment was analyzed as a split-plot design, with the water regime as the main plot effect and genotypes as the subplot effect, whereas the rain-fed experiment was analyzed as an alpha lattice. The statistical analysis was performed using GenStat software. The GGE-biplot analysis was performed with PBTools (2014).
3. Results and Discussion
- The weekly rainfall from June to October in the three environments is shown in Figure 1. There was a clear gradient in the amount of rainfall received; South Gedaref had high rainfall (400 mm), El Obeid had intermediate rainfall (288 mm), and Khartoum North had low rainfall (42 mm). The flowering occurred from 15 to 27 October at the three environments, and as there was no rain after October (week 20; Fig. 1) we confirmed that the plants were exposed to post-flowering drought at the three environments.
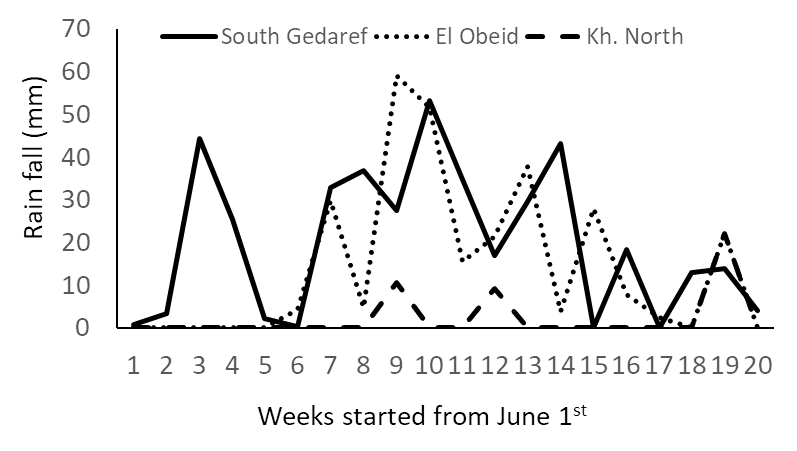 | Figure 1. Weekly rainfall from June to the end of October in Khartoum North, South Gedaref and El Obeid |
3.1. Performance under Imposed Drought at Khartoum North
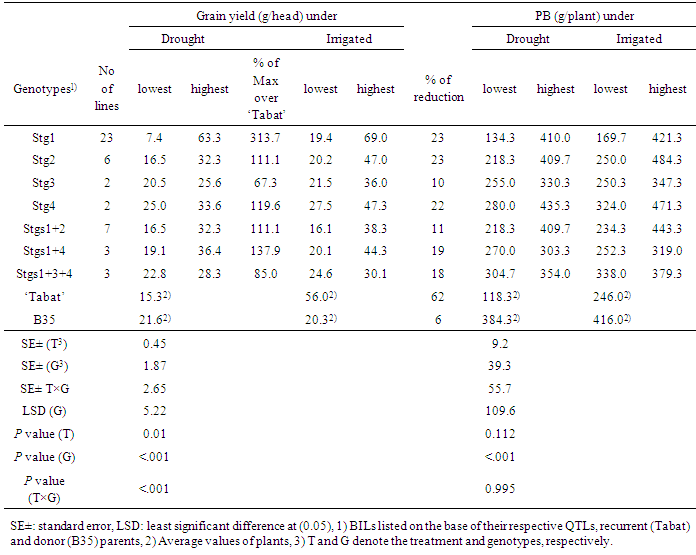
- Table 1 shows highest and lowest GY and PB per plant in each of the BILs seven groups with similar QTL combination and their parents under normal (irrigated) and drought conditions. Under irrigation, lowest GY of all the BILs was significantly less than the average of ‘Tabat’ and comparable to B35. The BILs with Stg1 showed significantly higher GY (69 g) than ‘Tabat’ and at least one line in each group had GY higher than B35 (Table 1).
|
3.2. The Performance of the Stay-green BILs under Rain-fed Conditions
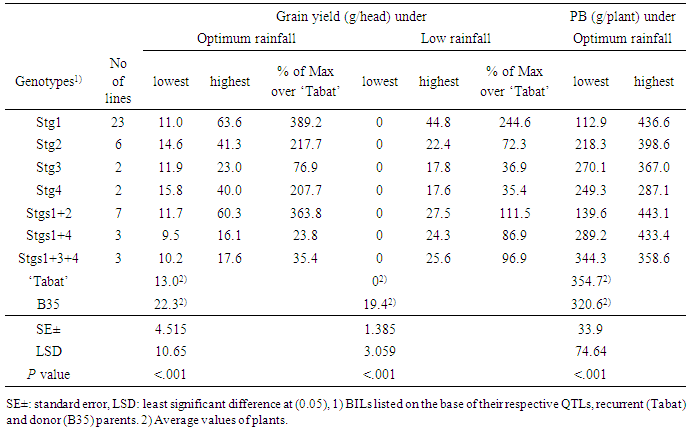
- Genotypes differed significantly (P< 0.001) for all scored traits both at a given rain-fed environment and across the two environments (Tables 3, 4). The two rain-fed environments (South Gedaref and El Obeid with relatively optimum and low rainfall, respectively) also differed significantly (P<0.001) for all traits and the genotype × environment interactions were significant (P<0.05). The parents had comparable GY at South Gedaref, while ‘Tabat’ had no GY due to the severe drought at El Obeid (Table 3). Under optimum rainfall, some BILs with Stg1, Stg2, Stg4 and Stg1+2 had higher GY than ‘Tabat,' whereas, at low rainfall, all the BILs out yielded ‘Tabat’ which had no GY (Table 3). Among the BILs, lines with Stg1 and Stg1+2 had higher GY than the other BILs under optimum rainfall, whereas only lines with Stg1 showed higher GY than the other BILs under low rainfall. Some BILs with a single QTL showed better performance than those with two and three QTLs under optimum rainfall, however, under low, rainfall, BILs with two and three QTLs did better than those with single QTL. The percentage of increase in GY of the BILs over ‘Tabat’ ranged from 389% in BILs with Stg1 to 24% in Stg1+4 and from 244% in BILs with Stg1 to 35% in Stg4 at the optimum and low rainfall environments, respectively (Table 3).
|
|
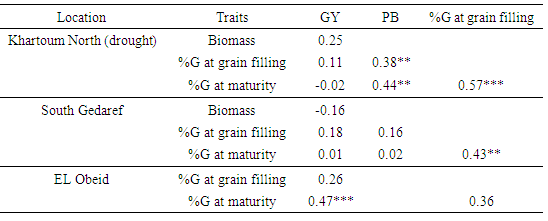
3.3. Simple Correlation Analysis
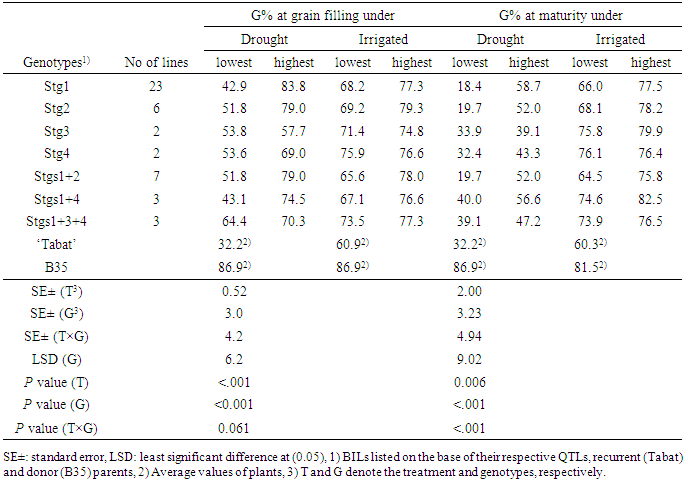
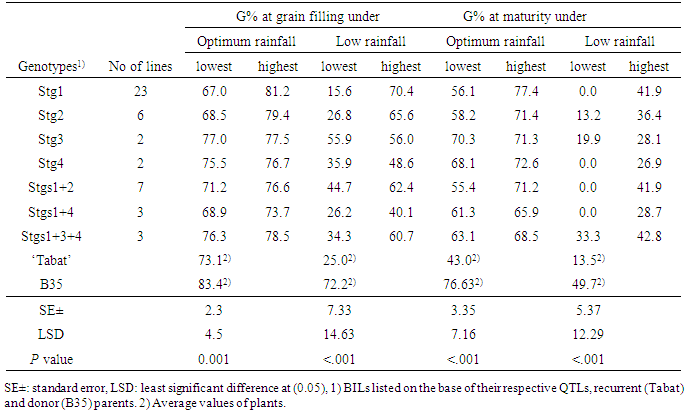
- For the three drought environments (Khartoum North, South Gedaref, and El Obeid), we calculated the correlations between GY, PB and the stay-green indicator %G. In Khartoum North (drought condition) and South Gedaref, GY did not correlate with PB and %G, whereas, in El Obeid, GY was positively correlated with %G at maturity (Table 5). Plant biomass correlated positively with %G at both grain filling and maturity at Khartoum North but not at South Gedaref. Percent greenness at grain filling correlated positively with %G at maturity at Khartoum North and South Gedaref but not at El Obeid. These results suggest that %G at both grain filling and maturity could be used as a selection criterion for higher grain yield and tolerance under post-flowering drought.
|
3.4. Variability of the Grain Yield and Stay-green in the BILs
- In order to investigate the variability of the performance of the BILs in terms of GY and stay-green, we performed GGE-biplot analysis. PC1 and PC2 explained 67% of the total variation for GY (Fig. 2a). The genotype comparison biplot showed that many BILs had better GY and were more stable than the recurrent parent ‘Tabat’ (G48) and the donor parent B35 (G1) (Fig. 2a). The best genotype was G8 carrying Stg1 followed by several BILs with Stg1, Stg2, Stg1+2 and Stg1+3+4. In the biplot of the stay-green indicator %G at maturity, PC1 and PC2 explained 90.8% of the total variation. None of the BILs outperformed the donor parent B35 (G1), the most stable and the greener genotype, whereas ‘Tabat’ (G48) was the most senescent genotype (Fig. 2b). The first three BILs followed B35 were with Stg1. It is worth noticing that the best genotype in term of GY (G8) with Stg1 also displayed lower rate of senescence consistently over locations. Generally, some of the genotypes that showed high GY also showed a good degree of %G compared to the recurrent parent ‘Tabat’. These results indicated the improved BILs performance and enabled us to see the progress achieved in the increase of the GY and %G of ‘Tabat’ especially across the drought environments. Based on the biplot analysis, several genotypes could be selected for advancement to further backcrossing. The results suggested that the performance of the BILs with Stg1 was better than the other BILs. Stg1 was the best in term of tillering reduction, water use and grain yield [16, 17]. On the other hand, Vadez et al. [15] reported that Stg1 contribute to reduction of tillering and leaf area and increase of water extraction under post-flowering drought depending on the genetic background. These reports and our results indicate the uniqueness of this QTL and its importance in breeding stay-green sorghum. In this study also we could shed light on the effect of the stay-green QTL pyramiding on sorghum grain yield under terminal drought the thing that is not extensively studied. Although we have few combinations our findings suggest that Stg1 is an important QTL and should be incorporated in any QTL combination.
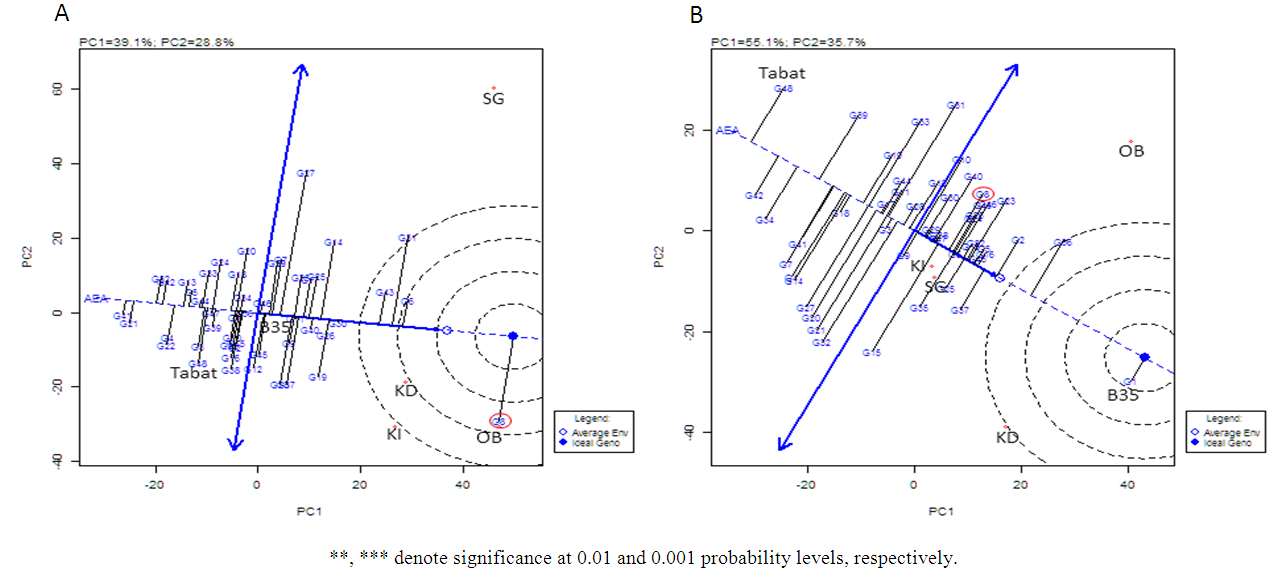 | Figure 2. GGE biplot of genotypes for grain yield (A) and percentage greenness (B) showing the best genotype based on mean performance and stability across the four environments. KD and KI denote drought and irrigated environments at Khartoum, respectively, SG and OB denote South Gedaref and El Obeid respectively. The best genotype G8 is indicated by circles |
4. Conclusions
- In this study, we examined progress made in incorporating stay green trait from B35 to ‘Tabat’ and the performance of 46 BC2F4 derived lines with one to three of the four putative stay-green QTLs. A reasonable degree of success has been achieved in our study, as a number of the QTL introgression lines were significantly more stay-green than ‘Tabat’, and able to better maintain their relative yield level in the post-flowering stress environments. Our results indicated that QTL pyramiding is essential to obtain better tolerance under post-flowering drought. However, this might not always be necessary. The QTLs significantly enhanced the GY under post-flowering drought, and the marker-assisted backcross scheme was effective. Superior lines identified will be selected for further backcrossing to restore all the yield potential and quality background of the recurrent parent ‘Tabat’ to produce high yielding, good quality and post-flowering drought tolerant sorghum cultivars suitable for cultivation under drought conditions in Sudan and similar agro-ecological environments.
ACKNOWLEDGEMENTS
- The authors greatly acknowledged Dr. Hanan A. Suliman and Dr. Elnayer H amid, El- Gedaref Research Station and Dr. Elgailani A. Abdalla, El Obeid Research Station for the great support and assistance during the experiments at their respective site.
Supplementary
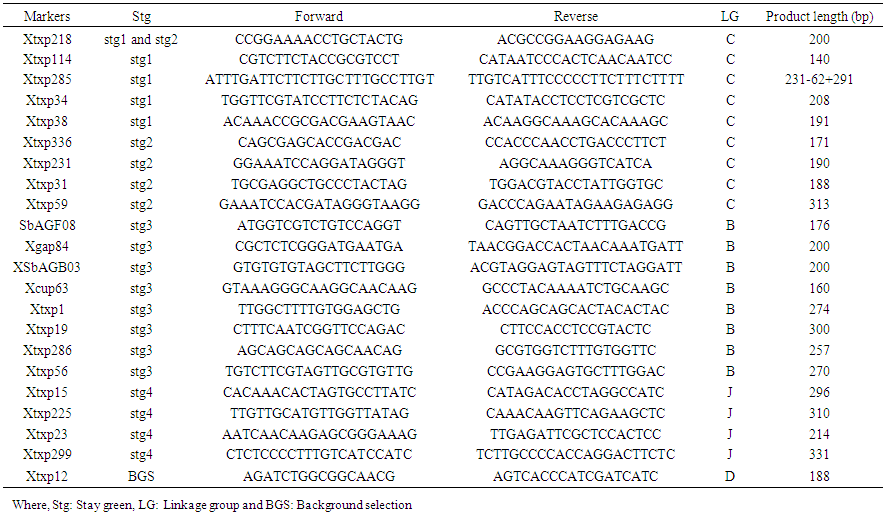 | Table S1. The twenty-two SSR markers selected from the consensus map of sorghum (Bhattramakki et al. 2000) and used for the marker assisted backcrossing to transfer the four stay-green QTLs from B35 to Tabat |
 | Figure S1. Schematic diagram explain the marker asissted backrossing steps followed to transferre the stay green QTLs from B35 to Tabat |
References
| [1] | Patrick, O. O., Volenec, J. J., Ejeta, G., 2016, Selection for drought tolerance in Sorghum using desiccants to stimulate post-anthesis drought stress, Field Crops Research 198, 312–321. |
| [2] | Ibrahim, N. E., and Abbas E. M., 2006, Experience of sorghum and millet production in Africa, a paper presented in eastern and central Africa regional sorghum and millet network of ASARECA (ECARSAM), Machakos, Kenya, 24th – 28th July. |
| [3] | Rosenow, D. T., and Clark L. E., 1981, Drought tolerance in sorghum. In: Proc 36th Annual Corn and Sorghum Res. Conf, December 9–11, 1981, Chicago, Illinois, pp 18–30. |
| [4] | Rosenow, D.T., Quisenberry, J. E, Wendt, C. W., Clark, L. E., 1983 Drought tolerant sorghum and cotton germplasm, Agric. Water Manage., 7, 207-222. |
| [5] | Crasta, O. R., Xu, W., Rosenow, D. T., Mullet, J., Nguyen H. T., 1999, Mapping of post-flowering drought resistance traits in grain sorghum: association between QTLs influencing premature senescence and maturity, Mol. Gen. Genet., 262, 579-588. |
| [6] | Rosenow, D. T., Ejet, G., Clark, L. E., Gilbert, M. L., Henzel, R. G., Borell, A. K., Muchow, R. C., 1996, Breeding for pre- and post-flowering drought stress resistance in sorghum. In: The international conference on genetic improvement of sorghum and pearl millet, September 23-27, 1996, Lubbock, Texas, pp 400-411. |
| [7] | Subudhi, P. K., Crasta, O. R., Rosenow, D. T., Mullet, J. E., Nguyen, H.T., 2000, Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor L. Moench), Genome, 43, 461-469. |
| [8] | Tao, Y. Z., Henzell, R. G., Jordan, D. R., Butler, D. G., Kelly, A. M., McIntyre, C. L., 2000, Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments, Theor. Appl. Genet, 100, 1225-1232. |
| [9] | Tuinstra, M. R., Grote, E. M., Goldsbrough, P. B., Ejeta, G., 1997, Genetic analysis of post-flowering drought tolerance and components of grain development in Sorghum bicolor (L.) Moench, Mol. Breed., 3, 439–448. |
| [10] | [Xu, W., Subudhi, K., Crasta, O. R., Rosenow, D. T., Mullet, J. E., Nguyen, H. T., 2000, Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor L. Moench), Genome, 43, 461–469. |
| [11] | Walulu, R. S., Rosenow, D.T., Wester, D. M., Nguyen, H. T., 1994, Inheritance of stay-green trait in sorghum, Crop Sci., 34, 970–972. |
| [12] | Kassahun, B., Bidinger, F. R., Hash, C. T., Kuruvinashetti, M. S., 2010, Stay-green expression in early generation sorghum [Sorghum bicolor (L.) Moench] QTL introgression lines, Euphytica, 172, 351–362. |
| [13] | Jordan, D. R., Hunt, C. H., Cruickshank, A. W., Borrell, A. K., Henzell, R. G., 2012, The relationship between the stay –green trait and grain yield elite sorghum hybrid grown in a range of environments, Crop Sci., 52, 1153-1161. |
| [14] | Reddy, N. R. R., Ragimasalawada, M., Sabbavarapu, M. M., Nadoor, S., Patil, J. V., 2014, Detection and validation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35, BMC Genomics, 15, 909. |
| [15] | Vadez, V., Deshpande S. P., Kholova, J., Hammer, G. L., Borrell, A. K., Talwar, H. S., Hash, C. T., 2011, Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background, Functional Plant Biology, 38, 553–566. |
| [16] | Borrell, A. K., van Oosterom, E. J., Mullet, J. E., George-Jaeggli, B., Jordan, D. R., Klein, P. E., Hammer, G. L., 2014, Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns, New Phytologist, 203, 817-830, doi:10.1111/ nph.12869. |
| [17] | Borrell, A. K., Mullet, J.E., George-Jaeggli, B., van Oosterom, E. J., Hammer, G. L., Klein, P. E., Jordan, D. R., 2014, Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake, J. Exp. Bot., 65, 6251-6263. |
| [18] | Beyene, A., Shimelis, H., Tongoona, P., Laing, M., 2015, Physiological mechanisms of drought tolerance in sorghum, genetic basis and breeding methods, Afr. J. Agric. Res., 10, 3029-3040. |
| [19] | Salih, A. A., Ali, I. A., Lux, A., Luxova, M., Cohen, Y., Sugimoto, Y., Inanaga, S., 1999, Rooting, water uptake, and xylem structure adaptation to drought of two sorghum cultivars, Crop Sci., 39: 168-173. |
| [20] | Kamal, N. M., and Ali, A. M., 2007, Marker assisted transfer of stay green trait to enhance terminal drought tolerance of the local sorghum cultivar Tabat. JSPS/JST international symposium on: toward advanced use of African resources in plant science. RIKEN, Yokohama, Japan. |
| [21] | Mahalakshmi, V., and Bidinger, F. R., 2002, Evaluation of stay-green sorghum germplasm lines at ICRISAT, Crop Sci., 42, 965-974. |
| [22] | R. Mustafa “Risk management in the rain-fed sector of sudan: case study, Gedaref area eastern Sudan” Dr. Agr. thesis, Justus-Liebig University, Giessen, Germany May 2006. |
| [23] | Khanna-Chopra, R., and Viswanathan, C., 1999, Evaluation of heat stress tolerance in irrigated environment of T. aestivum and related species. I. Stability in yield and yield components, Euphytica, 106, 169–180. |
| [24] | Thomas, H., and Rogers, L. J., 1990, Turning over an old leaf, Univ. Wales Rev. Sci. Technol., 6, 29-38. |
| [25] | Borrell, A. K., Bidinger, F. R., Sunitha, K., 1999, Stay-green associated with yield in recombinant inbred sorghum lines varying in rate of leaf senescence, Intl. Sorghum and Millets Newsl., 40, 31–33. |
| [26] | Sanchez, A. C., Subudhi, P. K., Rosenow, D. T., Nguyen, H. T., 2002, Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor (L.) Moench), Plant Mol. Biol., 48, 713–726. |
| [27] | Subudhi, P. K., Magpantay, G., Rosenow, D. T., Nguyen, H. T., 1999, Mapping and marker-assisted selection to improve stay green trait in sorghum for drought tolerance. In: Ito, O., Otoole, J. C., Hardy, B., (eds.) Genetic improvement of rice for water-limited environments. Los Banos, Philippines, pp 183–191. |
| [28] | Borrell, A. K., and Hammer, G. L., 2000, Nitrogen dynamics and the physiological basis of stay-green in sorghum, Crop Sci., 40, 1295–1307. |
| [29] | Duncan, R. R., Bockholt, A. J., Miller, F. R., 1981, Descriptive comparison of senescent and nonsenescent sorghum genotypes, Agron. J., 73, 849–853. |
| [30] | McBee, G. G., 1984, Relation of senescence, nonsenescence, and kernel maturity to carbohydrate metabolism in sorghum. In: Mughogho, L. K., (ed.) Sorghum root and stalk rots, a critical review. Proceeding of consultative group discussion of research needs and strategies for control of sorghum root and stalk diseases. ICRISAT, Patancheru, AP, India, pp 119–129. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML