-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2017; 7(3): 59-64
doi:10.5923/j.plant.20170703.01

Total alkaloids Antibacterial Activity in Vitro of Zanthoxylum madagascariense (Rutaceae) on Methicillin-Resistant Staphylococcus aureus and Enteropathogenic Escherichia coli to Hospitalized Patients
Rakotozafy J. C. R.1, Rasolohery C. A.2, Ratalata R. A. B.3, Ralaibia B. E.4, Rakotoarimanana R. A.1, Rajaonarison J. L.1, Rajaonarimamy E.1, Randrianananjato M. T.1, Razakarivelo J. F.4, Randriantsoa A.5
1Laboratory of Molecular Biology, University of Fianarantsoa, Madagascar
2Laboratory of Plant, Health, Fianarantsoa, Madagascar
3Laboratory of Natural Substance Chemistry, University of Fianarantsoa, Madagascar
4Laboratory of Chemistry, University of Fianarantsoa, Madagascar
5Laboratory of Molecular Pharmacology, University of Antananarivo, Madagascar
Correspondence to: Rakotozafy J. C. R., Laboratory of Molecular Biology, University of Fianarantsoa, Madagascar.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

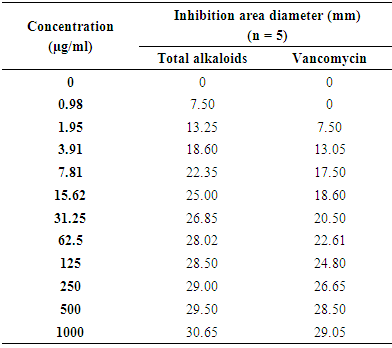
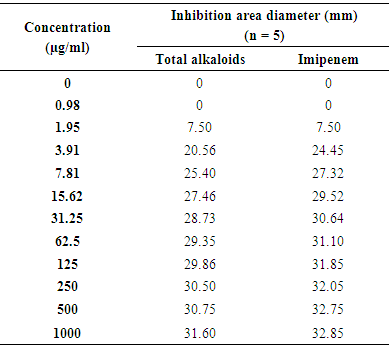
The antibacterial activity in vitro of total alkaloids to Zanthoxylum madagascariense (Rutaceae) has been compared with the one of vancomycin and imipenem on Methicillin-Resistant Staphylococcus aureus (MRSA) and Enteropathogenic Escherichia coli (EPEC), bacteria responsible of the nosocomial infection to the hospital. This extract is more active on MRSA than on EPEC. The inhibition zones diameter in 1.95 µg/ml are 13.25 mm and 7.5 mm respectively. To 125µg/ml this activity is higher compared to vancomycin in which their inhibition zones diameter are 28.50 mm and 24.80 mm respectively and lower to imipenem with 29.86 mm and 31.85 mm. In order to increase the antibacterial products panoply, chemical and pharmacological depth study of total alkaloids proves to be necessary.
Keywords: Total alkaloids, Zanthoxylum madagascariense, MRSA, EPEC, Fianarantsoa, Madagascar
Cite this paper: Rakotozafy J. C. R., Rasolohery C. A., Ratalata R. A. B., Ralaibia B. E., Rakotoarimanana R. A., Rajaonarison J. L., Rajaonarimamy E., Randrianananjato M. T., Razakarivelo J. F., Randriantsoa A., Total alkaloids Antibacterial Activity in Vitro of Zanthoxylum madagascariense (Rutaceae) on Methicillin-Resistant Staphylococcus aureus and Enteropathogenic Escherichia coli to Hospitalized Patients, International Journal of Plant Research, Vol. 7 No. 3, 2017, pp. 59-64. doi: 10.5923/j.plant.20170703.01.
Article Outline
1. Introduction
- The bacteria multi resistant to antibiotics had an important impact to hospital, allying a high mortality and to an important financial burden [1]. The multi resistance is a step toward the therapeutic dead end. It concerns the bacteria responsible for communal and nosocomial infections or associated to the cares. Among all multiresistant bacteria, Methicillin-Resistant Staphylococcus aureus (MRSA) and Enteropathogenic Escherichia coli (EPEC) are the most worrisome considering their pathogenic power, of their diffusion within the hospitals and to the community. Furthermore, these bacterial species can colonize the patient long time after getting out the hospital that can contribute to their dissemination within the general population [2]. These two bacterial stumps have an important place to nosocomial infections in the industrialized countries [3].In developing countries, the difficulties met in the struggle against the multi resistant bacteria are aggravated by poverty. Some infectious homes persist within the most underprivileged population. They are difficult to neutralize because of the both reasons scientific and economic. Besides, the antibiotics are not always available, or they are too expensive.Therefore, 80% of the population use plants to take care of themselves [4-6].Indeed, the antibacterial activity survey of total alkaloids of Zanthoxylum madagascariense (Rutaceae) has been done. The ethnopharmacologic investigation in the South-Center region of Madagascar revealed that this plant is used empirically to treat the different infectious illnesses of bacterial origin.
2. Materials and Methods
2.1. Sites of Study
- The study has been achieved in the patients at the Resuscitation Service to the Center Hospital University of Fianarantsoa (Madagascar). The choice of the service is based on the bacterial contamination high risk.
2.2. Withdrawal of the Samples
- Different samples have been collected with sterile recipient. The withdrawals have been done before antibiotics treatment. The appropriated samples have been sent to the laboratory for bacteria isolation and identification.
2.3. Bacteria Identification
- Staphylococcus aureus and Escherichia coli are the most frequently isolated and identified pathogenic bacterial to the hospitalized patients. Indeed, the study was focused on these kinds of bacteria. All withdrawals don't possess one of these bacterial species are excluded. Bacteria identification and antibiogram have been achieved by biomedical laboratory usual technique [7-10].
2.3.1. Media Culture
- A preliminary identification by Gram coloration (Kit Gram-Nicolle RAL®) has been achieved for every sample, to be able to classify the bacteria in cocci and bacillus group. According to the results of Gram methods, different culture medium are used to isolate the bacteria:- Staphylococcus aureus: on salty media (Chapman, BioRad®), some yellow colonies were looked for after 24 hours of 37°C incubation. - Escherichia coli: the sample is sowed on media (UriSelect, BioRad®) to isolate Escherichia coli. Purple colonies were looked for on culture medium after 48 hours of 37°C incubation.
2.3.2. Biochemical Test (API-System)
- API System (bioMérieux®) is a standardized system for genera bacteria identification, which uses miniaturized biochemical tests and a specially adapted database. The complete list of those bacteria that is possible to identify with this system can be found in the Identification Table at the end of this package insert. The API System strip consists of 20 microtubes containing dehydrated substrates. These microtubes are inoculated with a bacterial suspension, prepared in API System Medium that reconstitutes the tests. During incubation, metabolism produces color changes that are either spontaneous or revealed by the addition of reagents. The reactions are read according to the Reading Table and the identification is obtained by referring to the Analytical Profile Index or using the identification software [11].
2.3.3. PCR Procedure
- 2.3.3.1. DNA Extraction The DNA is extracted by Sambrook and col. Technique [12]. Staphylococcus aureus and Escherichia coli adjusted to 104 bacteria/ml have been put in culture in 5 ml of soya trypticase bouillon. After 37°C incubation during 24 hours, the culture has been centrifuged to 12 000g during 10 min. Then, the cheek bacterial has been washed with 500µl extraction tampon (TE) (10 mM Tris-HCl, pH 7.5 and 1 mM EDTA). The whole has been centrifuged again to 12 000g during 10 mn. After centrifugation, the cheek was resuspended in 200µl cellular lyse tampon (10 mMTris-HCl pH 7.5, 1 mM EDTA pH 7.9, 0.5% Tween 20) with 15U lysostaphin, incubated to 37°C during 1 hour. Then, 15µl of proteinase K (20 mg/ml) have been added to the suspension, then incubated to 56°C in 1 hour. Once all proteins are digested, the proteinase K has been deactivated by heating to 95°C during 15 mn. After the enzyme deactivation, the same volume of phenol-chloroform has been added in the mixture and centrifuged to 12 000g in 10 mn. The supernatant has been transferred in another tube and mixed with two volumes of 95% of ethanol. The whole has been frozen during one night at - 20°C. The following day, the suspension has been centrifuged to 12 000g in 5 mn. The cheek that contains the DNA has been washed with 70% of ethanol, centrifuged and dried. The DNA has been put in suspension in 100µl of sterile extraction tampon. The concentration as well as the DNA quality are controlled by DNA migration on agarose frost to 2%. The DNA stock has been kept to - 20°C.2.3.3.2. Methicillin-Resistant Staphylococcus aureus (MRSA)Ÿ rDNA 16S, mec A and nuc genes research by PCR Specific fragments research of rDNA 16S, nuc and mec A genes of MRSA have been done [13-16].The gene amplification has been done by Mastercycleur (Life Pro Thermal Cycler BIOER) with 25µl mixture of 300ng DNA extract, 1µl (50pmol) of each/ rDNA16S, nuc and mec A primers, 0,5μl (50μM) of mixture dNTPs, 2,5μl of the 1 X PCR tampons and 1 μl (3U) of DNA Taq Polymérase (PCR Amplification Kit: Geneshun Biotech Ltd). 2.3.3.3. Enteropathogenic Escherichia coli (EPEC)Ÿ rDNA 16S and eae genes research by PCR rDNA 16S and eae specific fragment research has been achieved. The gene amplification has been done with the help of a mastercycleur (Life Pro Thermal Cycler BIOER) with 25µl mixture of 300ng DNA extract, 1µl (50pmol) of each/ rDNA and eae primers, 0.5µl (50mM) of dNTPs mixture, 2.5µl of the 10X PCR tampons and 1µl (3U) of DNA Taq Polymerase [DEC PCR® Kit heart PCR detection of diarrhoeagenic E. coli (DEC), Denmark]. The mixture adjusted to 25µl with bidistilled water has been amplified. Aliquots (10 μL) of each PCR product were resolved by electrophoresis in a 1% of agarose gel stained with ethidium bromide. The amplified products were identified by UV irradiation of the gels and photographed by UVP® Transilluminator: BIODOC-IT IMAG SYSTEM (fig 1, 2). M: DNA molecular weight marker (ΦX174 DNA/HaeIII Marker). Lane 1, 2 and 6: Agarose gel electrophoresis showing the result of amplification of mec A gene (533pb), nuc gene (279pb) and rDNA 16S gene (750pb). M: DNA molecular weight marker (ΦX174 DNA/HaeIII Marker). Lane 1, 4 and 7: Agarose gel electrophoresis showing the result of amplification of rDNA 16S gene and eae gene (750pb).
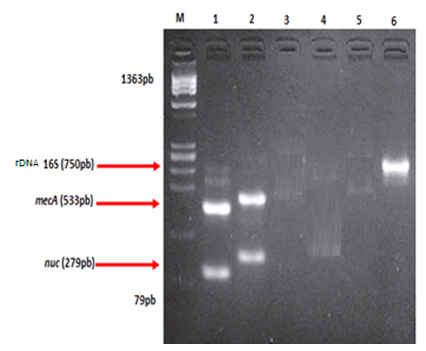 | Figure 1. Agarose gel electrophoresis showing the result of multiplex PCR for detection of Methicillin – Resistant Staphylococcus aureus |
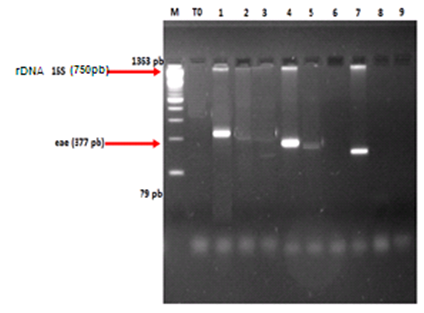 | Figure 2. Agarose gel electrophoresis showing the result of multiplex PCR for detection of Enteropathogenic Escherichia coli |
2.4. Total Alkaloids Extract
- Zanthoxylum madagascariense B. (Rutaceae), was collected from Fianarantsoa (Madagascar) and was authentically identified at Botanical and Zoological Tsimbazaza Park, Antananarivo (Madagascar).Dried and powdered stem bark (600g) was exhaustively extracted by maceration with acetic acid solution (pH = 4). The filtered solution alkalinised with NaHCO3 up to pH = 8 was then partitioned with CH2Cl2. The organic fraction was evaporated to dryness under reduced pressure to afford a total alkaloids extract. TLC analysis by Dragendorf reagent spraying revealed the presence of five major alkaloids.
2.5. Antibiotics Reference
- The results of the bacterial ecology survey in the hospital inert environment by Rakotozafy and col. in 2016 show that among the antibiotics tested on Staphylococcus aureus and Escherichia coli, Vancomycine® and Imipenem® are respectively sensitive [17]. Indeed, these two antibiotics have been chosen like antibiotic reference.
2.6. Extract Preparation
- Total alkaloids solubilized in 50mM phosphate tampon, containing 0.001% of DMSO (pH 7). From 2000µg/ml stock solution, 1000µg/ml to 0.12µg/ml concentrations (dilution 2 to 2) have been prepared.
2.7. Antibiotic Preparation
- 0.2g of antibiotic powder (Vancomycin® or Imipenem®) has been dissolved in 2 ml of sterile distilled water, filtered then aspirate in a syringe of 10 ml and filtered.
2.8. Antibacterial Disk Susceptibility Tests
- Antibiotic and total alkaloids extract susceptibility testing by a standardized single disk method have been used [7-10]. Four milliliters of this bacterial suspension (1-3 X 104 bacteria/ml) have been spread uniformly on the Mueller Hinton agar media (BioRad®). Afterwards, the different antibiotics and extract disks (Cypress Diagnostics®) have been deposited on media. After 48 hours of 37°C incubation, the inhibition area diameter has been measured. Every antibiotic and total alkaloids extract has been tested 5 times. The results are expressed in millimeter (mm).
2.9. Statistical Analysis
- The statistical analysis of the qualitative and quantitative variables was either of mono-varied type, or of multivariate type using a software Epinfo version 6. The Student test was used for the averages and the variances comparison and the test of Chi-2 for the comparison between the rates and a significant value if p<0. 05.
3. Results and Discussions
3.1. Methicillin-Resistant Staphylococcus Aureus
- The results of MRSA inhibition growth by total alkaloids and Vancomycin® are presented on table 1.
|
3.2. Enteropathogenic Escherichia Coli
- The results of EPEC inhibition growth by total alkaloids and Imipenem® are presented on table 2.
|
4. Conclusions
- Zanthoxylum madagascariense (Rutaceae) has been selected for this work on the basis of Malagasy traditional medicine use for different infectious illnesses treatment. The antibacterial tests permitted to demonstrate that the total alkaloids, extracted of this plant, possesses an antibacterial activity. In perspective, the total alkaloids toxicological profile proves to be necessary to be able to study its antibacterial activity in vivo.
ACKNOWLEDGMENTS
- This work was made possible with the Laboratory of Molecular Biology, University of Fianarantsoa (Madagascar).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML