-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2017; 7(2): 39-47
doi:10.5923/j.plant.20170702.03

Chemosystematic Aspects of the Moraceae Family: Phenylpropanoids and Aromatic Polyketides
Adriana Lima de Sousa 1, Cibele Maria Stivanin de Almeida 2, Maria Auxiliadora Coelho Kaplan 3, Rodrigo Rodrigues de Oliveira 2
1Instituto Federal Fluminense Campus Campos Guarus, Campos dos Goytacazes, Brazil
2Laboratório de Ciências Químicas, Universidade Estadual do Norte Fluminense Darcy Ribeiro-UENF, Campos dos Goytacazes, Brazil
3Núcleo de Pesquisas de Produtos Naturais, Centro de Ciências da Saúde, Bl. H, Universidade Federal do Rio de Janeiro, Cidade Universitária, Rio de Janeiro, Brazil
Correspondence to: Adriana Lima de Sousa , Instituto Federal Fluminense Campus Campos Guarus, Campos dos Goytacazes, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study discusses the evolutionary status of Moraceae, from the perspective of chemical features of phenolic micromolecules. A chemosystematics analysis points to affinities between genera and tribe belonging to the Moraceae family, by correlation of the protection parameters of micromolecule hydroxyls resulting from the mixed pathway (acetate/shikimate) and the shikimate pathway. The phenylpropanoid and aromatic polyketide hydroxyl groups are mainly protected by prenylation and methylation mechanisms. A chemometric analysis (grouping and factor analyses) was used to evaluate the evolutionary relationships of the Moraceae genera and tribe, and it was possible to establish taxonomic relationships for the systematic characterization of the Moraceae family through the chemosystematic data. The results of the chemosystematic study suggest evidence that the Trilepisium genus is inadequately classified in the Dorstenia tribe and that the Streblus genus does not belong to the Moreae tribe. In addition, this chemosystematic study confirms the advanced status of Moraceae and legitimization of intrafamiliar classification.
Keywords: Moraceae, Chemotaxonomy, Micromolecules, Mixed pathway, Shikimate pathway
Cite this paper: Adriana Lima de Sousa , Cibele Maria Stivanin de Almeida , Maria Auxiliadora Coelho Kaplan , Rodrigo Rodrigues de Oliveira , Chemosystematic Aspects of the Moraceae Family: Phenylpropanoids and Aromatic Polyketides, International Journal of Plant Research, Vol. 7 No. 2, 2017, pp. 39-47. doi: 10.5923/j.plant.20170702.03.
Article Outline
1. Introduction
- The Moraceae family consists in monophyletic taxa [1-3], constituted by a group of cosmopolitan species, comprising about 1500 species [4, 5]. According to APG IV (2016) [6], this family is classified as Rosales. Moraceae species present an impressive range of breeding systems and pollination syndromes, as well as enormous variations in growth [2-3, 7-9]. Furthermore, they present a great diversity of anatomical and morphological characteristics, in addition to floral complexity [4, 10].The intrinsic diversity of species belonging to the Moraceae family culminates in classification conflicts among its systematics, based on morphological and anatomical characters, as proposed by the researchers Rohwer (1993) and Berg (2001) [11-13] versus systematics based on evolutionary relationships and molecular phylogeny introduced by Dätwyler and Weiblen (2004), Beg (2005) and Clement and Weiblen (2009) [2, 3, 8]. The Moraceae systematic classification is still not resolved, due to suprageneric and infrageneric relationships.In view of this, we conjecture that the systematic classifications of the Moraceae family based only on morphological and phylogenetic data are insufficient to explain why this taxon “is related to mulberry and bread-fruit” [10]. There are still questions to be answered so that the evolutionary history of Moraceae can be traced. It has been questioned at what point in their history was insect pollination inserted [1-3, 5, 8, 12] and what are the geographical and temporal origins of this family [14-16].In this context, chemosystematics can contribute to the study of the positioning of Moraceae family genera as a conspicuous and complementary tool. Chemosystematics represents the integration of chemical data and organism morphology dependent on the association of genetic inheritance, and geographic and environmental regulators [17].The special metabolism stimulated along the angiosperm adaptive process as a defense subterfuge, consisting of micromolecules, is noteworthy, rich in structural diversity and biosynthesized in metabolic pathways derived from the primary metabolism [17, 18]. Chemical evolution in angiosperms is represented in terms of evolutionary channelling [19, 20], in which flavonoid biosynthesis developed prior to lignin biosynthesis [17]. The Moraceae metabolism is conspicuous in the production of metabolites from the mixed pathway (acetate/shikimate) compared to the shikimate pathway [21]. The high heterogeneity exerted by flavonoid derivatives corroborates Dalghren (1980) in his classification for Angiosperms [22].In angiosperms, evolutionary diversity is characterized by molecular protection processes in response to oxidative degradation [17-20, 23]. This occurs in the Moraceae family species concerning phenolic hydroxyls, resulting from the shikimate and the mixed pathway, alongside phenolic hydroxyls produced by methylation, glycosylation and prenylation [21].Moraceae species present abundant metabolites resulting from the mixed pathway, with unprotected oxylic groups. Prenylated flavonoids, such as flavone, flavonol, flavanone, chalcone, stilbene and diels alder adducts are noteworthy in this context.In the same way, micromolecules originating from the shikimate pathway, coumarins and lignans, have a majority of unprotected oxylic groups.Although this taxon does not present a key chemo marker, evolutionary advancement index evaluations provide valuable chemosystematic information.With this in mind, the chemosystematic aspects of phenylpropanoids and aromatic polyketides biosynthesized by Moraceae species were evaluated herein, according to the evolutionary advancement parameters regarding hydroxyl protection parameters. These data were used for similarity predictions between genera and in the understanding of Moraceae evolutionary chemistry.
2. Materials and Methods
2.1. Chemosystematic Methodology
- Chemical data were collected from an extensive literature survey regarding Chemical Abstracts, via Scifinder, covering the range from 1907 to 2014. Phenylpropanoids and aromatic polyketides identified in Moraceae species were listed and submitted to the chemosystematic methodology. It is worth noting that genera organization is based on recent phylogenetic studies conducted by taxonomists Dätwyler and Weiblen (2004) both specialists in Moraceae species.The evolutionary advancement indices for Moraceae genera related to phenylpropanoids and hydroxyl and aromatic polyketides hydroxyl protection mechanisms were determined as proposed by Gottlieb et al. [16] and Emerenciano [24]. These parameters can provide chemical advancements for Moraceae in relation to plant evolution. In addition, they also denote important ecological information regarding adaptive responses and point towards evolutionary trends. The chemical parameters for micromolecules resulting from the mixed and the shikimate pathways regarding O-glycosyl (AEG), O-methyl (AEM), O-prenyl (AEP), Total O-Protection (AEPT), as well as Total O-unprotection (AEUT), were calculated by Equations 1, 2, 3, 4 and 5, respectively.
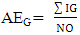 | (1) |
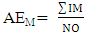 | (2) |
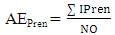 | (3) |
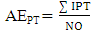 | (4) |
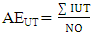 | (5) |
2.2. Multivariate Analysis
- In the present study the following statistical approaches were applied: factorial analysis and cluster analysis to explain observed similarities among Moraceae genera and tribes. The former provides tools to analyse the interrelationships (correlations) of a large number of variables, defining sets of variables which are strongly interrelated, known as factors (representing dimensions which summarize or explain the original set of observed variables). The main purpose of the second approach is to aggregate objects based on their characteristics, by recognizing and indicating relationship patterns [25]. The statistical analyses were conducted with Statistica® 7 for Windows.
3. Results and Discussion
- An estimated occurrence frequency of 404 phenylpropanoids and 1827 aromatic polyketides are listed in the Moraceae database. Moraceae metabolism shows a rich bioproduction of phenolic micromolecules preferably by the mixed (acetate/shikimate) pathway, instead of the shikimic acid derivative pathway, supporting the hypothesis that evolutionary channelling occurred in angiosperms. The occurrence frequency of these compounds in Moraceae genera is detailed in Tables 1 and 2. The chemometric exploration of evolutionary specialization and oxidation advancement parameters of phenylpropanoids and aromatic polyketides consisted in the observation of the similarities between genera, through a factorial and a cluster analysis. However, regarding hydroxyl protection, aromatic polyketides are mostly unprotected, and phenylpropanoids show peculiar protection patterns in each of the investigated genera. It must be considered that derivative protection systems must have evolved at the same time with micromolecular diversification. The hydroxyl protection indices of the evaluated genera are summarized in Table 3 and the hydroxyl protection indices of the tribes are displayed in Table 4.
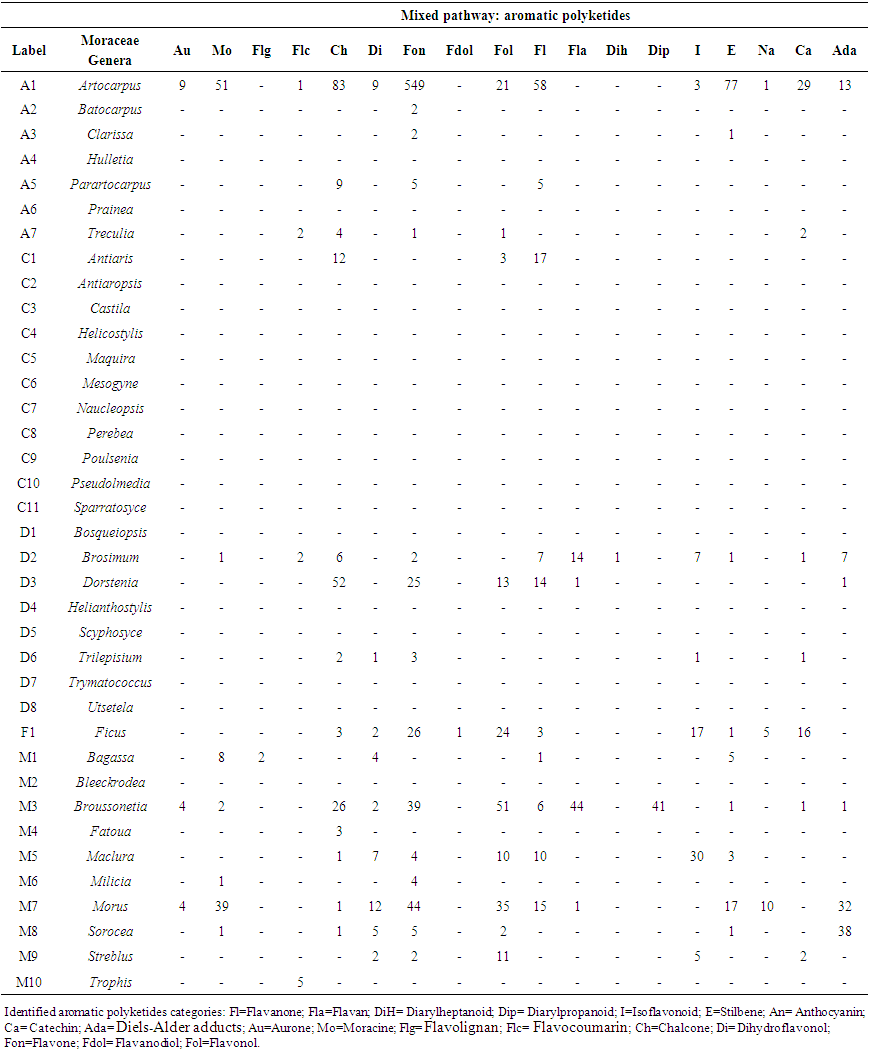 | Table 1. Occurrence frequency of aromatic polyketides in Moraceae genera |
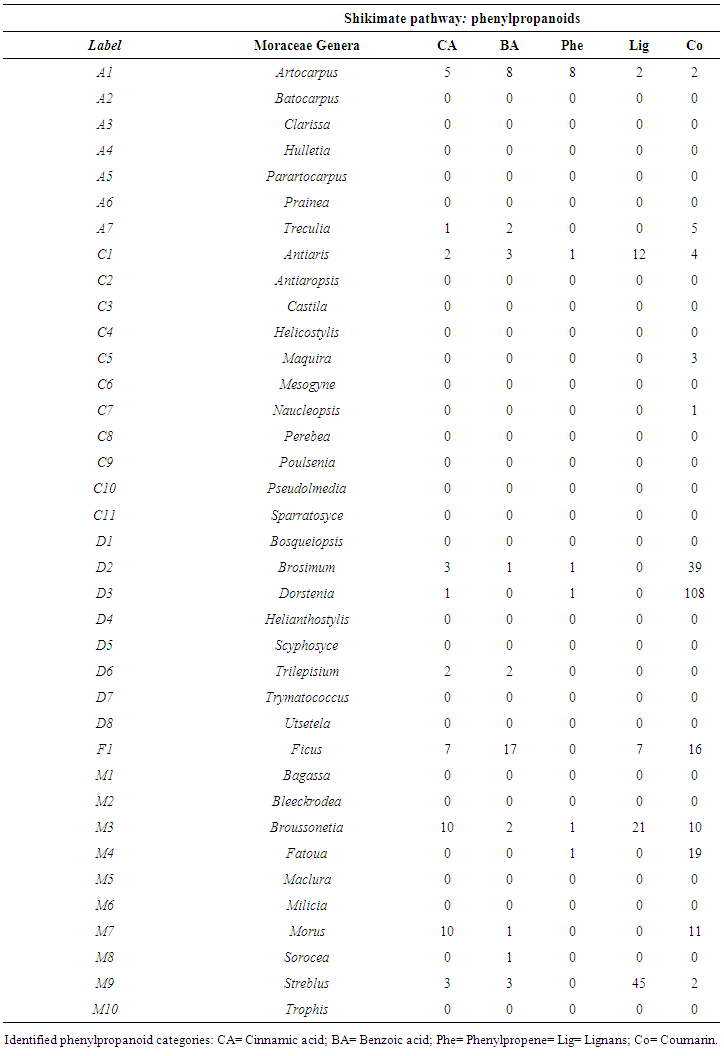 | Table 2. Occurrence frequency of phenylpropanoids in Moraceae genera |
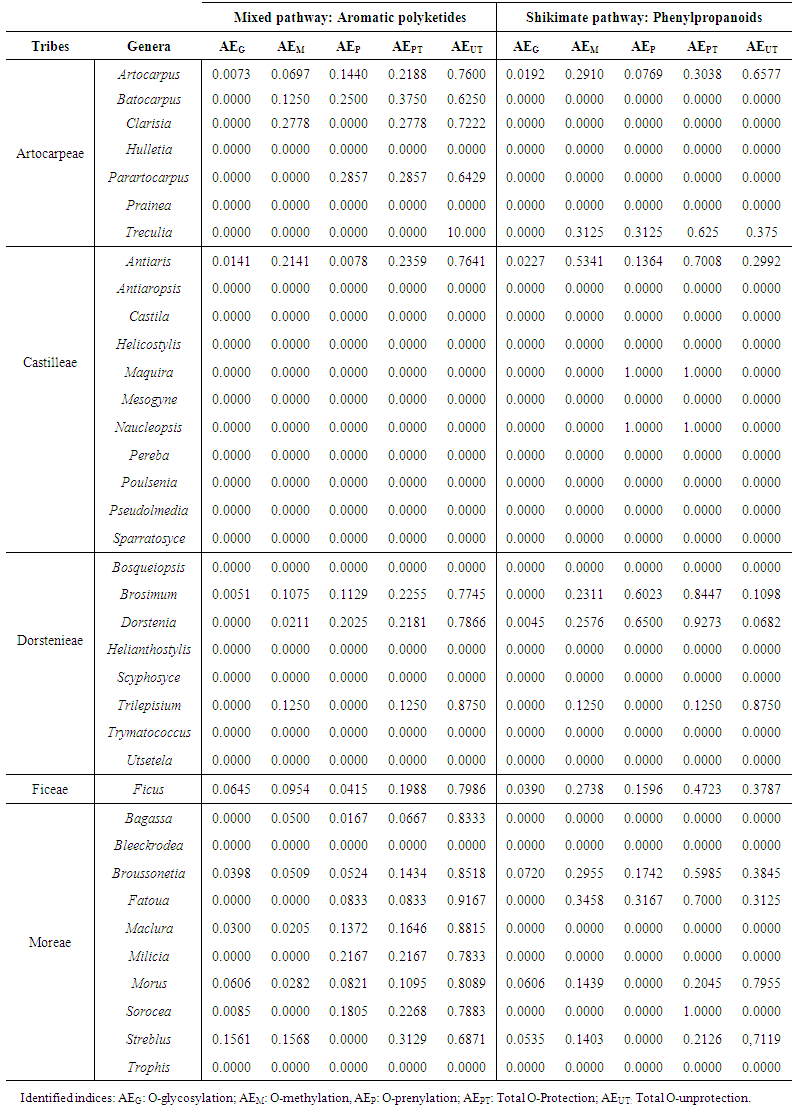 | Table 3. Values of the evolutionary protection and unprotection mixed pathway and shikimate pathway advancement parameters of Moraceae genera |
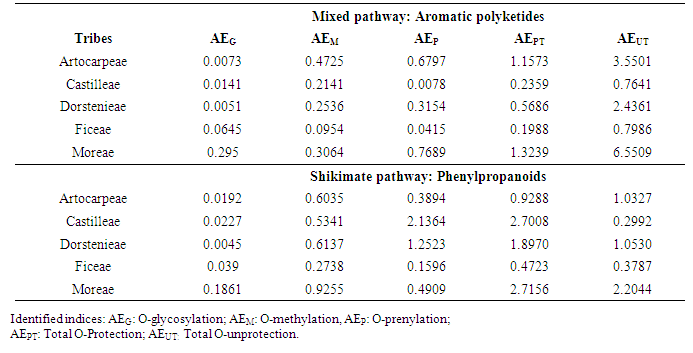 | Table 4. Values of the evolutionary protection and unprotection mixed pathway and shikimate pathway advancement parameters of Moraceae tribes |
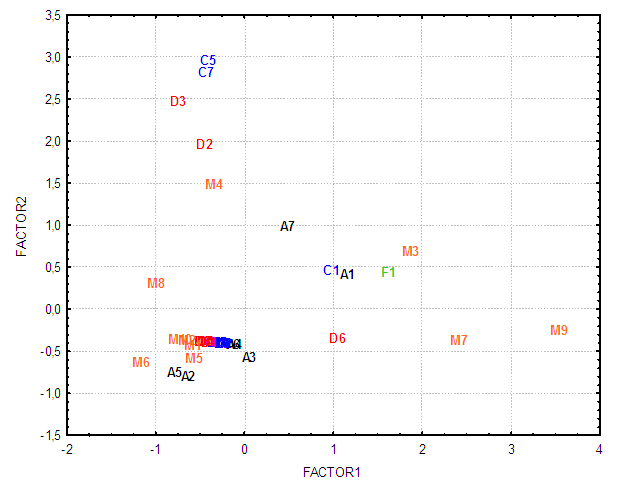 | Figure 1. Bidimensional diagram (Factor 1 x Factor 2) displaying the interrelationships between the 37 Moraceae family genera analyzed in the present study |
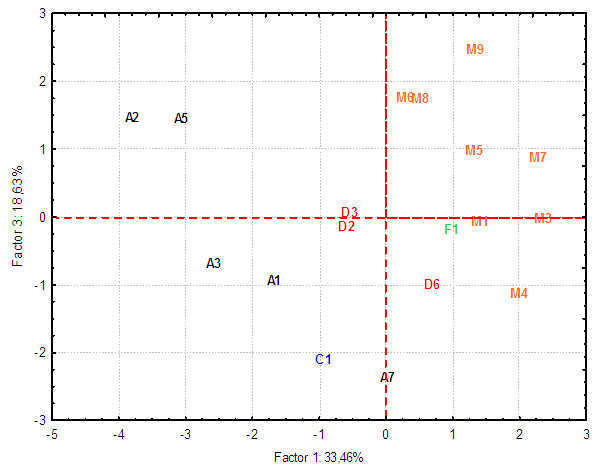 | Figure 2. Bidimensional diagram (Factor 1 x Factor 3) displaying the interrelationships between Moraceae family genera analysed in the present study |
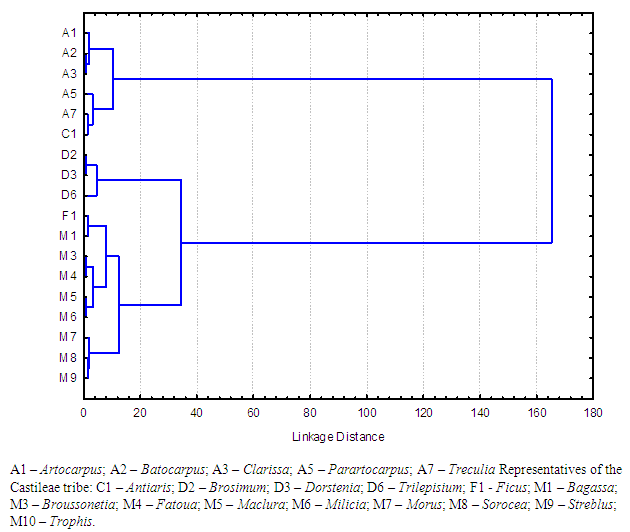 | Figure 3. Dendrogram of 37 Moraceae family genera analysed in the present study (Ward’s method based on Euclidean Distances) |
4. Conclusions
- This chemosystematic study greatly contributes to knowledge on the Moraceae family and ratifies that the evolutionary and oxidative advance parameters of Moraceae have systematic values.Analyses of the chemosystematic evolutionary advancement parameters of this family showed a high incidence of aromatic polyketides, with most hydroxyls unprotected, characterizing the primitive positioning of the Moraceae family. Phenylpropanoids have a high variety of hydroxyl protection and each genus showed a particular hydroxyl protection pattern.In sum, the chemosystematic data of phenolic micromolecules presented both similarities and dissimilarities between Moraceae genera and tribes. The main conclusions obtained herein were the consolidation that the Trilepisium genus forms a clade unlinked to the other genera of the Dorstenieae tribe, and that the Streblus genus is a discrepancy of the Moreae tribe and consist in a polyphyletic group.Chemosystematic studies, through the chemometric analysis of chemical evolutionary parameters can, thus, contribute greatly to the infrageneric classification of the Moraceae family. Even if the inventory of the Moraceae family is complemented and new data are aggregated, the evolutionary tendencies pointed out in this study will not be altered, due to the biosynthesis characteristics of special metabolites of each genus.
ACKNOWLEDGEMENTS
- The authors wish to thank UENF for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML