-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2017; 7(1): 12-20
doi:10.5923/j.plant.20170701.03

Morphophysiological Changes in Cenchrus ciliaris and Digitaria commutata Subjected to Water Stress
Taoufik Amari1, Issam Saidi2, Manel Taamali1, Chedly Abdelly1
1Laboratoire des Plantes Extrêmophiles, Centre de Biotechnologie de Borj-Cédria, Hammam-lif, Tunisia
2Faculty of Sciences of Gafsa, Unit of Macromolecular Biochemistry and Genetic, Zarroug, Gafsa, Tunisia
Correspondence to: Taoufik Amari, Laboratoire des Plantes Extrêmophiles, Centre de Biotechnologie de Borj-Cédria, Hammam-lif, Tunisia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Among abiotic stresses, water stress is the most environmental constraint for crops, especially in the arid and semi-arid tracts of the world. It reduces growth and development that may cause diverse disturbance in physiological, biochemical and structural integrity of plants. In this view, we assess here drought-induced changes in Cenchrus ciliaris and Digitaria commutata. Seedlings were grown under two watering regimes for three months. Water stress significantly restricted the photosynthesis and growth activity of both species. Interestingly, water deficit led to a slight decrease of relative water content (RWC). The maximal quantum yield of PSII photochemistry (Fv/Fm) remained unchanged. However, in light-adapted leaves, water deficit reduced the efficiency of excitation energy capture by open PSII reaction centers (Fv’/Fm’) and the quantum efficiency of the photosystem II (ΦPSII), increased the non-photochemical quenching (qN) and showed no effects on the photochemical quenching (qP). These results suggest that C. ciliaris and D. commutatashowed a better aptitude to preserve the PSII functional integrity, thereforea relatively good tolerance to water deficit.
Keywords: Cenchrus ciliaris, Chlorophyll fluorescence, Digitaria commutata, Photosystem, Water stress
Cite this paper: Taoufik Amari, Issam Saidi, Manel Taamali, Chedly Abdelly, Morphophysiological Changes in Cenchrus ciliaris and Digitaria commutata Subjected to Water Stress, International Journal of Plant Research, Vol. 7 No. 1, 2017, pp. 12-20. doi: 10.5923/j.plant.20170701.03.
Article Outline
1. Introduction
- Nowadays, the shortage of water is a serious world-wide problem and it is expected that climate change will accelerate the severity of droughts. UN reports (2006) [1] estimate that one third of world population has been living in areas where the water sources are poor. During the last few decades, water has become an increasingly scarce and precious resource, caused by worldwide climate change and increase in world population, the availability of water will have a greater impact on our ability to produce crops today [2]. Plants, as one of basic food sources, require adequate soil moisture to grow normally and complete its life cycle. It is well known that water stress is one of the most environmental factors limiting crop production worldwide [3]. The water scarcity is the major constraint affecting the survival, growth and plant development [4]. Among the likely mechanisms for decreasing plant development is related to a restriction of photosynthesis, respiration, ion uptake and translocation, as well as the nutrients metabolism and plant growth regulators [5, 6]. Many studies have shown that the photosynthesis reduction under drought can be linked with the biochemical processes disruptions [7]. A previous works demonstrated that water stress has a deleterious effect on the oxygen-evolving complex of photosystem (PSII) [8] and to the PSII reaction centers [9]. PSII photochemistry is hardly affected by water stress [10].Cenchrus ciliaris and Digitaria commutata are perennial grasses common on the arides zones of Tunisia [11]. These species constitute a most important food crop for animals in the pastures. Owing the high degree of complexity in interactions of various factors affecting plant growth, these species would be threatened with extinction. Therefore, it is important to evaluate the impact of water scarcity on plant life.In this work, we assess the effect of water stress on Cenchrus ciliaris and Digitaria commutata. A particular attention was paid to plant growth parameters, photosynthetic activity (assessed by gas exchange and chlorophyll fluorescence).
2. Materials and Methods
2.1. Soil Characteristics
- The soil used in this study was collected from the horizon 0–20 cm depth from Gafsa, a city in the south-west of Tunisia. The following soil properties were determined: pH (in water) 7.4; K+ (0.41 μequiv. g−1soil); Na+ (1.21 μequiv. g−1soil); Ca2+ (244.69 μequiv. g−1soil); electric conductivity EC (96.68 μs cm−1); organic matter content (0.87%). Five kilogram of dried soil was maintained in an imperforated plastic pot and saturated with tap water for 48 hours. The pot was covered with aluminum foil to prevent loss of water by evaporation. After two days of desiccation in an oven at 100°C, the soil field capacity was measured. The field capacity is the water content held in the soil after 48 hours of dripping of the free water.
2.2. Plant Material and Stress Treatments
- In this study, seeds of Cenchrus ciliaris and Digitaria commutata both species were collected near the National Park of Bouhedma (449 km east of Tunis). Seeds were germinated in plastic pots containing 32 kg of substrate soil (3 plants per pot). Four weeks after germination, the pots were covered with aluminum foil to prevent loss of water by evaporation, thereby accounting for the water lost only by leaf transpiration. Then, 24 plants were subjected to a controlled irrigation treatment (70% of field capacity) for 90 days, which led to substrate water deficit and the other 24 plants to field capacity. The experiment was conducted for a period of three months and it carried out in an open-air area under natural light and ambient temperature, in order as to keep all plants under conditions as similar as possible to those in the field.
2.3. Water Relations
- Leaf water potential (ΨW) was measured in three randomly selected leaves per plant per treatment, using a pressure chamber model 1000 (PMS Instrument Company, USA). Leaf relative water content (RWC) was measured according to Nauš et al. (2016) [12]. For this purpose, five discs were removed from mature leaves, immediately weighed to obtain fresh mass (MF) and placed under water in the dark for 12 h until full rehydration. The discs were weighed again to obtain the turgid mass (MT) and placed in a forced ventilation oven at 75°C until constant dry mass (MD). From these variables, the relative water content (RWC) was calculated as:[RWC = ((MF-MD) / (Mt-MD)) x100]Instantaneous and intrinsic water use efficiencies were estimated as the ratios of Pn and E of Pn and gS, respectively.
2.4. Growth Parameters
- The height, total leaf number and leaf area of all plants were evaluated weekly. Individual leaf area (LA) was estimated from sum of measurements of the length of the midrib (L) and maximum width (W) of each leaf, which were used in the equation LA = (LW)0.9660 suggested by Pompelli et al. (2012) [13]. The results were summed to obtain the total leaf area.At the harvest, plants (6 replicates per treatment) were then divided into roots and shoots. Roots were successively rinsed three times with cold distilled water and blotted between two layers of filter paper. The fresh weight was immediately estimated, and the dry weight was measured after 48 h of desiccation in an oven at 60°C.
2.5. Pigment Content
- Leaf chlorophyll concentration (6 replicates per treatment) was measured by the absorption spectra of frond extracts using a UV spectrophotometer. Three hundred milligrams of small discs from fresh leaves was extracted in 3 ml 80% acetone and absorbance of extracts was recorded at 470, 646.8 and 663.2 nm [14].
2.6. Photosynthetic Parameter Measurements
2.6.1. Leaf Gas Exchanges
- Leaf gas exchange variables were performed at 21, 42, 63 and 90 DAST in fully mature leaves (6 replicates per treatment) using a portable, open-system infrared gas analyzer LCi device under the following conditions: 398 ± 1 ppm CO2 concentration, 30 ± 0.3°C leaf temperature, and 1012 mBar atmospheric pressure. Net photosynthetic rate (Pn, µmol CO2 m−2s−1), stomatal conductance (gs, µmol H2O m−2s−1) and the transpiration rate (E, µmol H2O m−2s−1).
2.6.2. Chlorophyll Fluorescence
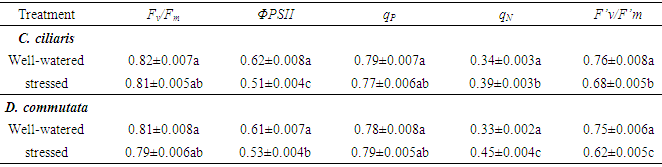
- Chlorophyll fluorescence measurements were also recorded fully mature leaves according to Genty et al. (1989) [15]. Measurements were taken using a portable modulated fluorimeter (PAM-2000, Walz, Germany). Leaves were dark-adapted for at least 20 min using leaf clips. The minimal fluorescence level (F0) with all PSII reaction centers open was measured by the measuring modulated light which was sufficiently low not to induce any significant variable fluorescence. The maximal fluorescence level (Fm) with all PSII reaction centers closed was determined by a 0.8 s saturating pulse at 8000 mmol m−2 s−1 in dark-adapted leaves. The leaf disc was then continuously illuminated with white actinic light at an intensity of 180 mmol m−2 s−1 which was equivalent to growth PPFD of wheat seedlings in the growth chamber. The steady-state value of fluorescence (Fs) was thereafter recorded and a second saturating pulse at 8000 mmol m−2 s−1 was imposed to determine the maximal fluorescence level in light-adapted leaves (Fm’). The actinic light was removed and the minimal fluorescence level in the light-Plant material and stress treatments adapted state (F0’) was determined by illuminating the leaf disc with 3 s far-red light. Then, the fluorescence parameters determined in both light and dark-adapted were used to calculate the maximal quantum yield of PSII photochemistry, (Fv/Fm), the photochemical quenching coefficient, qp= Fm’− Fs/(Fm’− F0’ ) and the non-photochemical quenching coefficient, qN = 1− (Fm’− F0’) /Fm − F0) which is linearly related to heat dissipation [16] the efficiency of PSII reaction centers, Fv’/Fm’ and the actual quantum yield of PSII electron transport, ΦPSII [16].
2.7. Statistical Analysis
- ANOVA with orthogonal contrasts and mean comparison procedures were used to detect differences between species and water regimes. Mean separation procedures were conducted using Duncan’s multiple range tests with least significant difference (LSD) (P < 0.05).
3. Results
3.1. Water Status
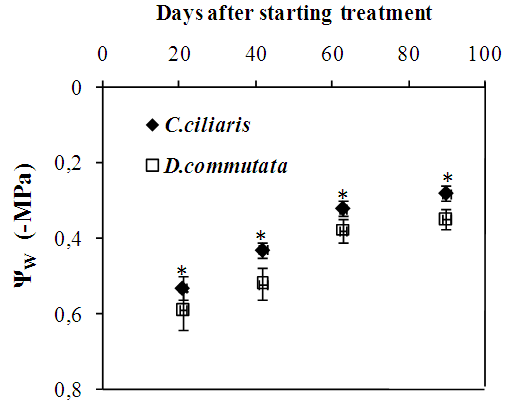
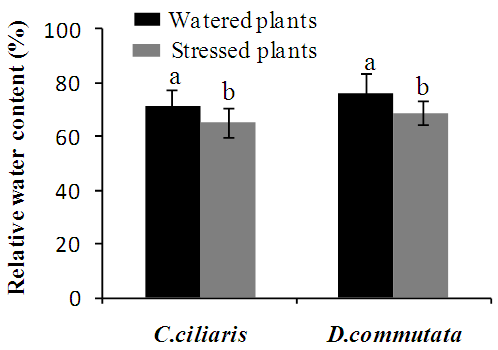
- A significant reduction (p<0.05) of the leaf water potential was observed from 21 days after starting treatment in water-stressed plants of Cenchrus ciliaris and Digitaria commutata (Fig.1). In both species, the relative water content of leaves water stress decreased slightly (Fig.2).
3.2. Gas Exchange
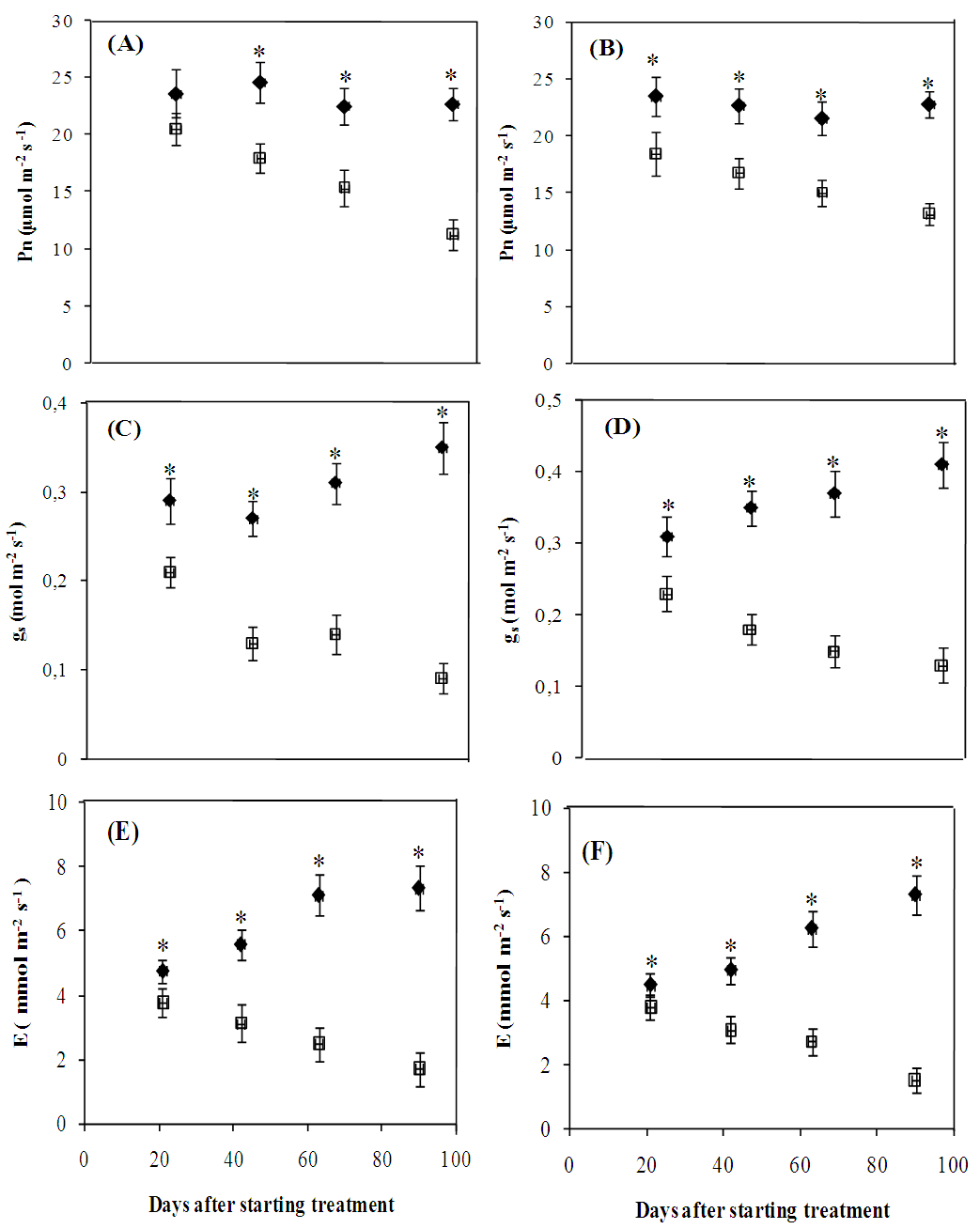
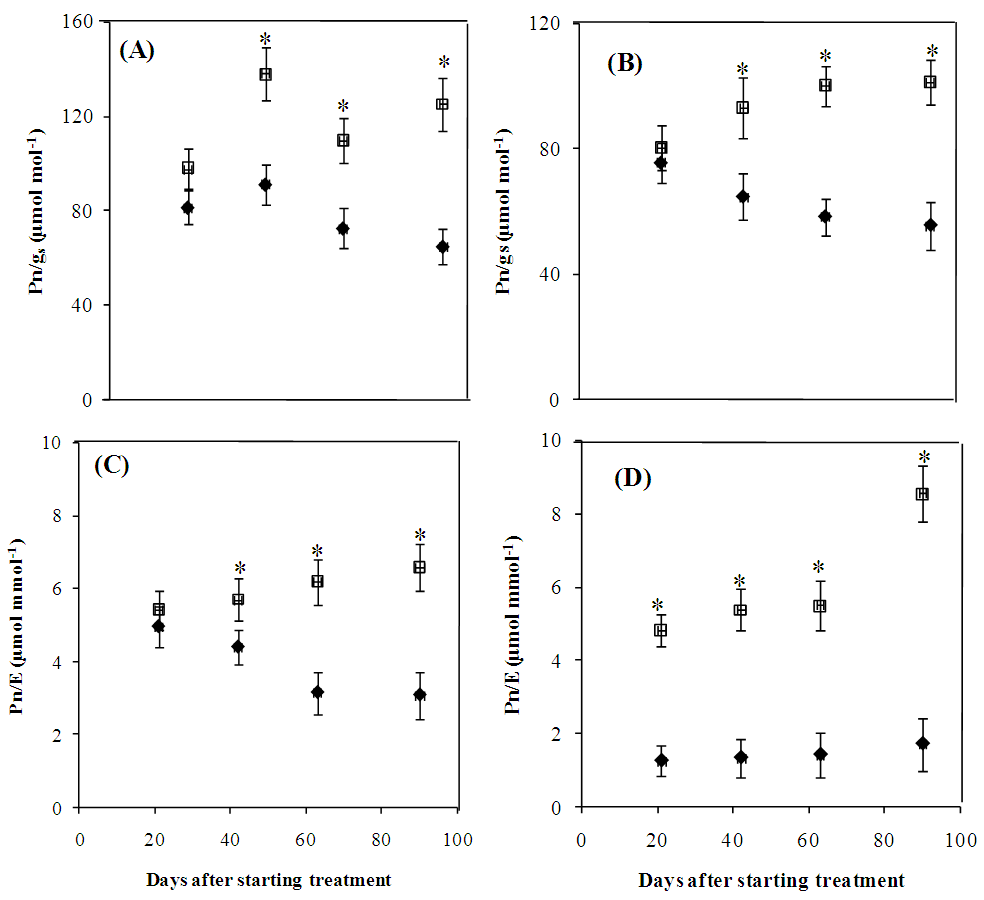
- The photosynthetic activity of water-stressed Cenchrus ciliaris and Digitaria commutata plants was adversely impacted as reflected by the significant decline of the main gas exchange parameters (Fig. 3). Net photosynthetic rate (Fig.3A-B), stomatal conductance (Fig.3 C-D) and the transpiration rate (Fig.3 E-F) were significantly (p<0.05) reduced in both species by the water stress treatment (Fig.3A-B-C). In Cenchrus ciliaris, Pn, gs and E values recorded at the 63th day were, respectively, 32, 55 and 65% lower than those of the control. For Digitaria commutata, water deficit led to Pn, gs and E reductions of 30, 59 and 56% respectively. There were no significant differences between species for the intrinsic (Pn/gs) and instantaneous (Pn/E) water use efficiencies (Fig. 4). A significant (p<0.05) increase of Pn/gs was observed in water stressed plants of both species (Figure 4A-B).The Pn/E shows a similar trend for both species, with a significant increase, especially at the 63 and 90th days after starting treatment (Fig. 4C-D).
3.3. Photosynthetic Pigments
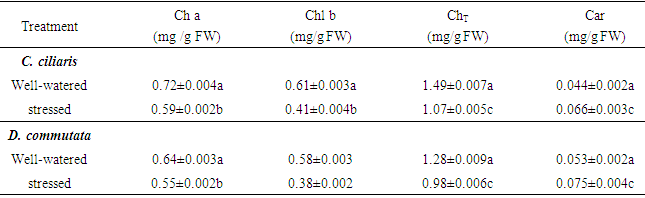
- Water stress decreased chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (ChlT) content in water-stressed Cenchrus ciliaris and Digitaria commutata plants (Table 2). This was associated with a significant increase of the carotenoid concentration, in both species, especially at the 42 and 63th days after starting treatment.
|
3.4. Growth
- Water stress exposure of Cenchrus ciliaris and Digitaria commutata plants induced early morpho-phytotoxicity symptoms. In both species, there was a general decrease in the shoot heights of the plants in the different water treatments (Fig. 5A). There was a significant difference (P<0.05) between the shoot height of the water-stressed plants and the well-watered plants from the beginning of the experiment until the last day of the experiment. Both root and shoot biomass decreased significantly in both species with increasing water deficit (Fig. 5B-C). As a result, the whole plant biomass production of both species was adversely affected by water stress (Fig. 5D). Numbers of leaves and leaf area were also significantly reduced in both species following water stress (Fig.5E-F).
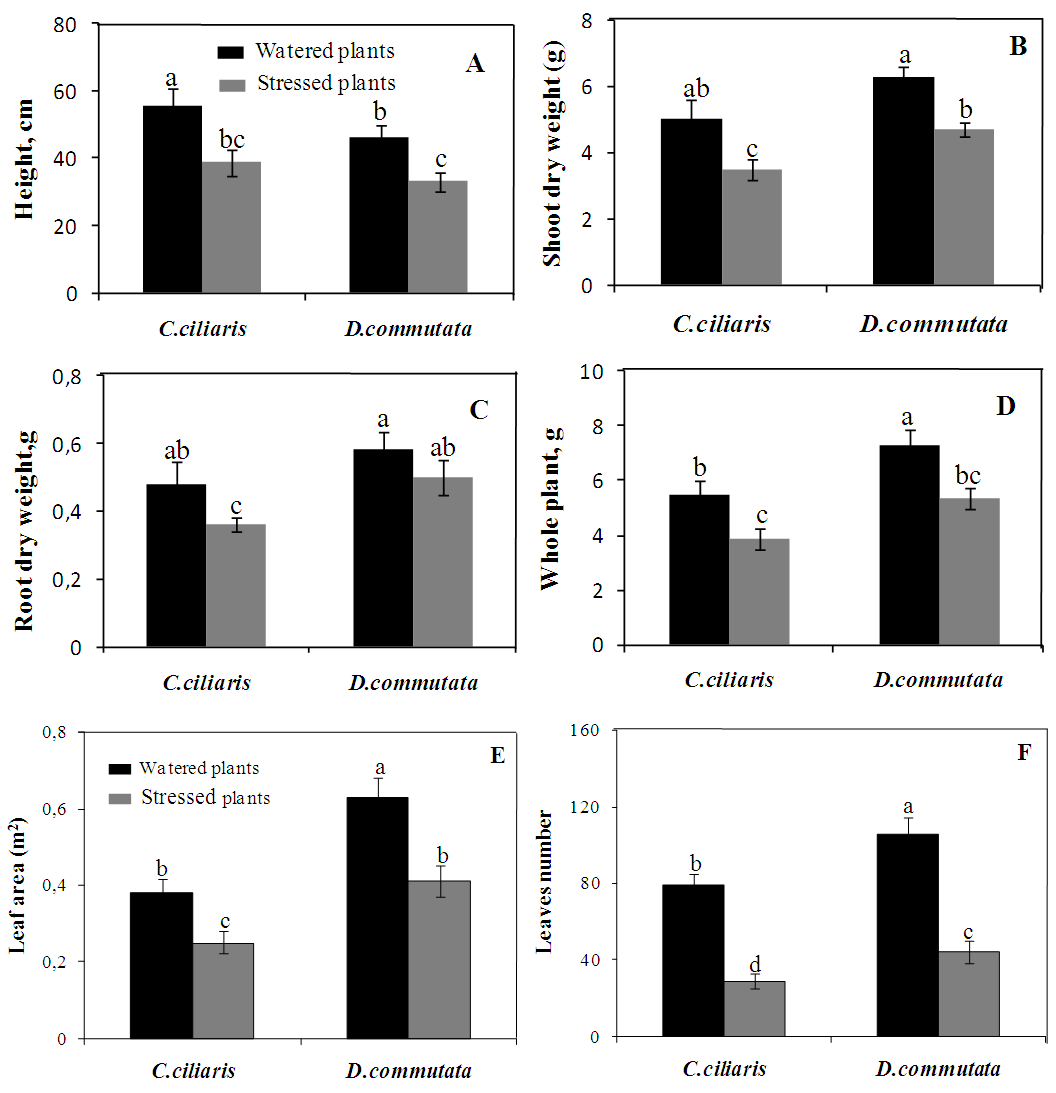 | Figure 5. Effect of water stress on the height (A), shoot biomass (B), root biomass (C) the whole plant dry weight (D), Leaf area (E) and leaves number (F) of Cenchrus ciliaris and Digitaria commutata. Means (n = 6 per treatment ± SE) with at least one same letter are not significantly different at P ≤ 0.05 |
4. Discussion
- Cenchrus ciliaris and Digitaria commutata has been described as being adapted to arid climates and dry-land agricultural ecosystems [11]. It is incontestable that under adequate soil moisture conditions, plants species will show higher productivity. Nevertheless, plants developed a wide range of strategies, at morphological, anatomical and cellular levels to cope with water stress [17]. Among mechanisms that allow the plant to avoid the stress or to increase its tolerance, the reduction of the leaf water potential is a common way maintaining cell function through elevated relative water content and stomatal closure [17]. In the present study, there is a slight decrease in both Ψw and the relative water content (Fig. 1 and 2). A similar result has been reported by Fini et al. (2013) [18] in Jatropha curcas exposed to water deficit. The conservation of the water content in C. ciliaris and D. commutata can be considered a strategy to avoid dryness in plant tissues. It was assumed that osmotic adjustment is responsible for maintaining an adequate RWC in plants [19]. The water use efficiency was increased in C. ciliaris and D. commutata plants (Fig. 4). This is consistent with other studies showing that maintain adequate water use efficiency under drought with several physiological traits could be linked to water and carbon balances [20]. Photosynthesis is vital metabolic process for plant development and productivity, which is known to be severely impacted by biotic and abiotic constraints. Gaining a better understanding of how environmental stresses affect the plant physiological status, either in field studies or under controlled conditions especially imposes a reliable diagnosis of the photosynthetic functioning. Continuous technical improvements allowed the utilization of simple, quick and non-destructive portable devices like Chlorophyll fluorescence and gas exchange [16]. Water stress is one of the most factors inhibiting photosynthesis [8, 10]. In our experiment, the decline of foliar photosynthetic rate (Fig. 3A-B) in C. ciliaris and D. commutata was associated with a parallel decrease in stomatal conductance (Fig. 3C-D). The transpiration rate decreased also significantly in both species (Fig. 3E-F). According to the literature, the simplest explanation for the reduction of photosynthetic activity during water stress would be that the stomatal conductance and the internal CO2 concentration decrease, following closure of stomata [21]. These authors have reported that stomatal conductance is more reduced when a plant is stressed. Photosynthesis decline under drought through metabolic impairment is a complex phenomenon than stomatal limitation [22]. It could be due to the biochemical processes perturbations [22]. Chlorophyll fluorescence data showed changes of photochemical activity in water-stressed plant of C. ciliaris and D. commutata (table 1). The maximal quantum yield of PSII photochemistry (Fv/Fm) remained almost constant during the time course of water stress treatment since Fv/Fm was close to 0.80, a value typical of healthy plants [16]. The quantum yield of PSII electron transport (ΦPSII) declined significantly. The decreased of ΦPSII could be due to the reduction in the efficiency of excitation energy capture by open PSII reaction centers (Fv’/Fm’) because of no change in photochemical quenching (qP) [15] (Table 1). According to Baker (1991) [23] the decrease in Fv’/Fm’ may reflect light-induced non photochemical quenching. Results show that water stress induced an increase in qN. This increased qN would dissipate some excitation energy at the photochemical utilization expense [24]. Therefore, high qN in water-stressed conditions was described in some plants as a regulatory mechanism to down-regulate photosynthetic electron transport so that production of ATP and NADPH would match with the decreased CO2 assimilation due to the closure of stomata [7].Photosynthetic pigments are important to plants mainly for collecting light and may be used as indicators of water stress damage. They may predict subsequent events at the organism level [21]. In this study, water stress produced changes in chlorophyll content (Chl a, b and ChT) (Table 2). The chlorophyll tissue concentrations were decreased in stressed plants of of C. ciliaris and D. commutata. Similar results have been reported in drought stressed Catharanthus roseus seedlings [5]. The water stressed plants of both species showed significantly higher carotenoids concentration as compared to the control (table 2). The increase of carotenoids content in plant was considered an important process to alleviate water stress [25]. It has been established that drought stress is a very important limiting constraint in plant growth and establishment. It greatly suppresses both elongation and expansion growth [4]. In the present study, water deficit negatively affected the plant growth in both C. ciliaris and D. commutata (Fig.5). Biomass productivity of shoots and roots, were significantly reduced in response to water stress, with shoot biomass being more impacted than root biomass (Fig. 5B-C). Significant reduction of the height plant was also observed (Fig. 5A). This result was similar to previous studies showing the decrease of the stem length and the plant biomass production under drought stress [26]. In our experiment, water deficit reduced numbers of leaves and in turn the leaf area (Fig. 5E-F). A similar trend was found by Akıncı and Lösel (2010) [6] in cotton plants and some Cucurbitaceae members. Although, the development of optimal leaf area is essential to photosynthesis and dry matter yield. Reduction in leaf area by rolling may also be important in controlling water loss and reflects changes in leaf turgor [27]. Leaf adaptations were considered a tolerance mechanism favoring the success of plants and allowing the resistance to drought [28]. Some authors pointed out that, in some species any reduction in cell size, due to loss of turgor during expansion, will lead to a higher stomatal frequency [29]. It is also worth mentioning that plants, under severe drought conditions, tend to develop morphological characters namely increases in proportion of leaf vein tissue compared to leaf area, increased stomatal numbers per unit of leaf surface, smaller sizes of stomata, epidermal and mesophyll cells, greater density of leaf hairs but smaller hairs, thicker outer epidermal walls and cuticle [30]. In addition, several physiological and biochemical mechanisms could govern drought tolerance in plants.
5. Conclusions
- Our finding indicated that water stress negatively affect the gas exchange and the growth activity of both C. ciliaris and D. commutata. Both species showed a better aptitude to preserve the PSII functional integrity when challenged with water deficit. The maintenance of an adequate relative water content and water potential suggests that both species are water savers. The morphological change, especially reduction of leaves numbers and leaf area, under water stress, can be also considered as an eventual strategy to control water loss.
ACKNOWLEDGEMENTS
- This work was conducted in the Extremophile Plant Laboratory, in the biotechnologie center of Borj Cedria and supported by the Tunisian Ministry of Higher Education and scientific research (LR10CBBC02).
References
| [1] | UN Human Development Report 2006. Beyond scarcity: Power, poverty and the global water crisis. Accessed: 8 August 2011. |
| [2] | McKersie, B., Planning for food security in a changing climate, 2015, J. Exp. Bot. eru., Vol.547. |
| [3] | Cousins, S.R., and Witkowski, E.T.F., African aloe ecology: a review, 2012, Vol. J. Arid Environ., 85, pp. 1–17. |
| [4] | Shao, H.B., Chu, L.Y., Shao, M.A., Abdul Jaleel, C., and Hong-Mei, M., Higher plant antioxidants and redox signaling under environmental stresses, Comp. Rend. Biol., 2008, Vol. 331, 433–441. |
| [5] | Jaleel, C.A., R. Gopi, B. Sankar, M. Gomathinayagam, and R. Panneerselvam. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress, C.R. Biol., 2008, Vol. 331, pp. 42–47. |
| [6] | Akıncı, S., and Lösel, D.M., The effects of water stress and recovery periods on soluble sugars and starch content in cucumber cultivars, Fresen. Environ. Bull., 2010, Vol. pp.19, 164–171. |
| [7] | Lu, C., and Zhang, J., Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants, J. Exp. Bot., 1999, Vol. 50, pp. 1199–1206. |
| [8] | Cornic, G., Drought stress and high light effects on leaf photosynthesis. In: Baker NB, Bowyer JR, eds. Photo- inhibition of photosynthesis: from molecular mechanisms to the field. Oxford, UK: Bios Scientific Publishers, 1994, Vol. pp. 297–313. |
| [9] | He, J.X., Wang, J., and Liang, H.G., Effects of water stress on photochemical function and protein metabolism of photosystem II in wheat leaves, Physiol. Plant, 1995, Vol.93, pp. 771–777. |
| [10] | Liang, J., Zhang, J., and Wong, M., Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynth. Res., 1997, Vol. 51, pp. 149–159. |
| [11] | Chaieb, M., and Boukhris, M. Flore succinte et illustrée des zones arides et sahariennes de Tunisie, l'Or du Temps 1998. |
| [12] | Nauš, J., Šmecko, S., and Špundová, M., Chloroplast avoidance movement as a sensitive indicator of relative water content during leaf desiccation in the dark, Photosynth. Res., 2016, Vol.129, pp. 217–225. |
| [13] | Pompelli, M., Antunes, W., Ferreira, D., Cavalcante, P., Wanderley-Filho, H., and Endres, L., Allometric models for non-destructive leaf area estimation of Jatropha curcas, Biomass Bioenerg., 2012, Vol.36, pp. 77–85. |
| [14] | Lichtenthaler, H.K., and Welburn, A., Determination of total carotenoids and chlorophylls a and b of leaf extract in different solvents, Biochem. Soc. Trans. 1983, Vol. 603, pp. 591–2. |
| [15] | Genty, B., Briantais, J.M., and Baker, N.R., The relationship between the quantum yield of photosynthetic electron and quenching of chlorophyll fluorescence, Biochim. Biophys. Acta, 1989, Vol. 99, pp. 87–92. |
| [16] | Maxwell, K., and Johnson, N., Chlorophyll fluorescence: a practical guide, J. Exp. Bot., 2000, Vol.51, pp. 659–68. |
| [17] | Fang, Y., and Xiong, L., General mechanisms of drought response and their application in drought resistance improvement in plants, Cell Mol. Life Sci., 2015, Vol.72, pp. 673–689. |
| [18] | Fini, A., Bellasio, C., Pollastri, S., Tattini, M., and Ferrini, F., Water relations, growth, and leaf gas exchange as affected by water stress in Jatropha curcas, J. Arid Environ., 2013, Vol.89, pp. 21–29. |
| [19] | Yooyongwech, S., T. Samphumphuang, R. Tisarum, C. Theerawitaya, and S. Cha-Um. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline, Sci. Hortic., 2016, Vol.198, pp.107–117. |
| [20] | Martínez-Vilalta, J., Poyatos, R., Aguadé, D., Retana, J., and Mencuccini, M., A new look at water transport regulation in plants, New Phytol., 2014, Vol.204, pp.105–115. |
| [21] | Farooq, M.A., Wahid, N., Kobayashi, D., Fujita, S.M.A., and Basra, Plant drought stress: effects, mechanisms and management, Agron. Sustain. Dev., 2009, Vol.29, pp.185–212. |
| [22] | Reddy, A.R., Chaitanya, K.V., and Vivekanandan, M., Drought induced responses of photosynthesis and antioxidant metabolism in higher plants, J. Plant Physiol., 2004, Vol.161, pp.1189–1202. |
| [23] | Baker, N.R., Possible role of photosystem II in environmental perturbations of photosynthesis, Physiol. Plant., 1991, Vol. 81, pp.563–570. |
| [24] | Brestic, M., Cornic, G., Fryer, M.J., and Baker, N.R., Does photorespiration protect the photosynthetic apparatus in French bean leaves from photoinhibition during drought stress? Planta, 1995, Vol. 196, pp.450–457. |
| [25] | Manoharan, P.T., V. Shanmugaiah, N. Balasubramanian, S. Gomathinayagam, M.P. Sharma, and K. Muthuchelian. Influence of AM fungi on the growth and physiological status of Erythrina variegata Linn. grown under different water stress conditions, Eur. J. Soil Biol., 2010, Vol.46, pp.151–156. |
| [26] | Wu, Q.S., R.X. Xia and Y.N. Zou, Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress, European J. Soil Biol., 2008, Vol.44, pp. 122–128. |
| [27] | Morgan, J.M., Differences in adaptation to water stress within crop species. Adaptation of plants to water and high temperature stress, (ed. by Neil C. Turner and Paul J. Kramer). 1980. pp. 369–382. John Wiley & Sons, New York. |
| [28] | Kummerow, J., Adaptation of roots in water-stressed native vegetation. Adaptations of plants to water and high temperature stress, (ed. by Neil C. Turner, Paul J. Kramer) 1980.pp. 57–73. John-Wiley & Sons, New York. |
| [29] | Fitter, A.H., and Hay, R.K.M., Environmental Physiology of Plants, Academic Press, 1987. London. |
| [30] | Akıncı, Ş., and Lösel., D.M., Plant water-stress response mechanisms, Water Stress, 2012, Vol. p.15–42. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML