-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2017; 7(1): 5-11
doi:10.5923/j.plant.20170701.02

Screening Kenya Local Coastal Maize Landraces for Resistance to Maize Weevil (Sitophilus Zeamais Motschulsky) and Larger Grain Borer (Prostephanus Truncates)
Ndiso J. B.1, Mugo S.2, Kibe AM3, Pathaka RS3, Likhayo P.4
1Pwani University, Kilifi, Kenya
2International Maize and Wheat Improvement Center, CIMMYT Kenya, Village Market, Nairobi, Kenya
3Egerton University, Egerton, Njoro, Kenya
4KALRO NARL, Nairobi, Kenya
Correspondence to: Ndiso J. B., Pwani University, Kilifi, Kenya.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Maize (Zea Mays L.) is the most important food crop in Kenya including coastal region. Kilifi and Kwale Counties account half of all the maize production in the region. The coastal region has food deficit because it produces 50,000 tonnes of maize per year, while the demand is 22 m tonnes. High poverty levels in the region are mostly due to low average maize yield as a result of low soil moisture availability due to low and erratic rainfall, and losses due to storage pests. Losses in maize grain yield which are caused by the maize weevil (Sitophylus zeamais M.) and the larger grain borer (LGB) (Prostephanus truncates H.) among post harvest pests ranges between 15 to 60%. Despite the availability of improved maize varieties in the coastal region, farmers still grow the local coastal maize landraces (LCML). The research was to study LCML in an effort to understand why farmers prefer to grow them in spite of released improved maize open pollinated varieties (OPVs) and hybrids. The objective was to screen for resistance to maize weevil and larger grain borer in 25 LCML and 5 improved checks. The screening was done in a laboratory at Kenya Agricultural Research Institute (KARI) Kiboko using complete blocks design (CBD). Data collected was on weight of ruminant grain (g), weight of flour (g) and per cent weight loss (%). From the comparisons of weight losses caused by both pests, it appears that weevils cause more damage than LGB. Maize weevil caused 12.2% (Kanjerenjere) - 32.4% (KDV-3) weight losses compared to only 5.0% (Chitweka) - 8.7% (Matsere) caused by the LGB. This implies that, given the same conditions, weevils are more disastrous and priority should be given to controlling them. There was variability for resistance to maize weevil and LGB in storage among Kenyan local coastal maize landraces. A pattern was identified whereby, landraces from Kwale, Kilifi and Lamu showed resistance to LGB. No such pattern was observed for the maize weevil where resistance was wide spread in the region. The length of exposure to maize weevil has been considerably longer than LGB, which was observed only in the early 1990s. The susceptibility of some improved varieties to storage pests may partly explain why farmers grow local cultivars since PH 4 is the most readily available commercial cultivar. Landraces with superior responses to storage pests were identified, and these may be used directly or as sources of resistance in various insect resistance breeding program objectives in coastal Kenya.
Keywords: Maize, Landraces, Storage pests, Weevils and large grain borer
Cite this paper: Ndiso J. B., Mugo S., Kibe AM, Pathaka RS, Likhayo P., Screening Kenya Local Coastal Maize Landraces for Resistance to Maize Weevil (Sitophilus Zeamais Motschulsky) and Larger Grain Borer (Prostephanus Truncates), International Journal of Plant Research, Vol. 7 No. 1, 2017, pp. 5-11. doi: 10.5923/j.plant.20170701.02.
1. Introduction
- Maize can store well when harvested at the right time and stored at the right moisture content less than 15% grain moisture content. Because most farmers store their maize while still in the cobs it is hard to bring the moisture content to less than 15%. Stored maize is normally kept above the cooking place to dry and prevent pest from attacking it. While the abiotic factors like drought stress cause loss in grain yield in the region, the biotic factors like storage pests cause heavy losses. Most farmers (70%) grow local coastal maize landraces (LCML) despite the improved varieties which have been released for growing in the region. There was need to screen the LCML for resistance to storage pests for conservation and breeding purposes. According to Gethi (2002) maize grain is subjected to infestation of a complex of pests consisting primarily of insects, mites and fungi, which contribute to post harvest losses. Post harvest insect pests are known to jeopardize food security throughout the developing world. Among the insects that destroy maize, the weevil (Sitophilus spp) and the LGB are most important in Kenya. They cause direct damage by feeding on stored grain and damage grain through physical deterioration by encouraging fungi development, thus reducing grain quality. Unfortunately traits that contribute to improved grain storage have been largely ignored (Bergvinson, 2000). Grain is most susceptible to maize weevil (Sitophilus zeamais Motschulsky) damage if it is stored at moisture content higher than 15% (CIMMYT, 2001). A consequence of the above facts is that maize weevil is a greater problem in developing than in developed countries. Maize weevil (Sitophilus zeamais Motschulsky) (Figure 1) is an important pest of stored maize in the tropics, particularly in the lowland and mid-altitude, hot, and humid environments (Longstaff, 1981; DeVries and Toenniessen, 2001; Pingali and Pandey, 2001). Studies in Malawi (Golob, 1984) and Zimbabwe (Giga et al., 1991) have reported >20% weight loss caused by weevils for untreated grain of maize hybrids stored in traditional structures, whereas up to an 80% loss may occur in on-farm stores in tropical countries (Mutiro, et al., 1992; Pingali and Pandey, 2001). Maize weevil damage maize kernels after dough stage. The maize weevil larvae feed on the kernel internally in the field and in storage (McMillian, et al. 1968). Weevil damage results directly in lost food and may also reduce future maize production for farmers who save grain as seed (a practice that accounts for 70% of all maize) planted in Eastern and Southern Africa (Pingali and Pandey, 2001). The use of pesticides for control of weevil is broadly recommended, but resource poor farmers of the developing world often cannot afford or obtain them. Also the increasing occurrence of insecticide resistance (Perez-Mendoze, 1999) and environmental concerns about use of chemical insecticides mean alternative control methods are required (Dhliwayo and Pixley, 2003). Use of resistant cultivars is the most promising method of minimizing damage due to S. zeamais where high input control measures such as pesticides and other integrated pest management (IPM) systems are difficult or unwise (Tiger, et al, 1994). Significant genetic variation for resistance to weevil has been found in several studies (Kim, et al, 2003).
 | Figure 1. Maize weevil |
 | Figure 2. Larger grain borer (LGB) |
2. Materials and Method
- Experimental siteThe screening was carried out at Kenya Agricultural Research Institute (KARI) Kiboko, Kenya. This is located at longitude 37.75oE and latitude 2.15oS, at an elevation of 975 meters above the sea level (ASL), receives 530 mm annual rainfall, maximum temperature were 35.1°C while minimum temperatures were 14.3°C and has predominantly sandy clay soils (Jaetzold et al., 2012). Experimental designMaize weevilComplete block design (CBD) was used. Maize kernels (from Morphological characterization trial at KARI Mtwapa) was ddisinfected of any field infestation of the maize weevil by heating in ventilated electrical oven a 60-70°C for 2 hours and the grain was left to cool in the oven overnight (used kilner jars with glass lids). Sterilized the jars in an electric oven set at 100°C for 15 minutes, together with their lids and wire gauze. This was aimed at controlling mites and other pathogens. Weighed 50g of each sample into the jars, replicated 3 times. Count 50 active adult insects and introduce into each jar. As a guide, use one insect per gram of host. Close the lids, arrange the jars in a complete randomized design, with the checks placed in such a way to take care of microclimate changes. Incubate (keep on the shelves) in the culture room where the relative humidity was 70% ±5% and temperature of 27°C ±2° for 10 days to allow oviposition. At the end of 10 days, remove the parents and take record of dead ones. Return the grains to respective jars. The jars are left in the incubation room for 90 days, while being rotated occasionally to minimize positional effects in the growth chamber. After 90 days, sieve the contents of each jar across a set of sieves (4.75 mm and 1 mm aperture) to separate grains from insects and flour from insects. Take record of weight o grain, flour (dust) and insect counts. Note. % dust produced = ((wt dust)/50g) x 100. Grain weight loss (%) = ((50 - wt of grain at 90 days)/ 50g) x 100 (CIMMYT, 1989).Lager grain borerComplete block design (CBD) was used. Maize grains was disinfected of any field infestation of the LGB pest by heating in ventilated electrical oven a 60-70°C for 2 hours and let the grain cool in the oven overnight (use kilner jars with glass lids). The jars were sterilized in an electric oven set at 100°C for 15 minutes, together with their lids and wire gauze. This is aimed at controlling mites and other pathogens. Weighed 50g each sample into the jars, replicated 3 times. Count 50 active insects and introduce into each jar. As a guide, use one insect per gram of host. Close the lids and incubate (keep on the shelves) in the culture room where the relative humidity was 70% ±5% and temperature of 27°C ±2° for 90 days. Arrange the jars in a complete randomized design, with the checks placed in such a way to take care of microclimate changes. After 90 days, sieve the contents of each jar across a set of sieves (4.75 mm and 1 mm aperture) to separate grains from insects and flour from insects. Take record of weight o grain, flour (dust) and insect counts. Note. % dust produced = ((wt dust)/50g) x 100. Grain weight loss (%) = ((50 - wt of grain at 90 days)/ 50g) x 100 (CIMMYT, 1989).Data collectionData collected was flour weight and grain weight loss (%) calculated from the difference of the weight before and after maize weevil/LGB infestation.Statistical analysisAnalysis of variance of resistance to storage pests (traits) was performed using general linear model (GLM) SAS computer package (Appendix 6). The means were separated using Duncan’s multiple range tests at 5% level of significance according to Steel and Torrie (1980).
3. Results
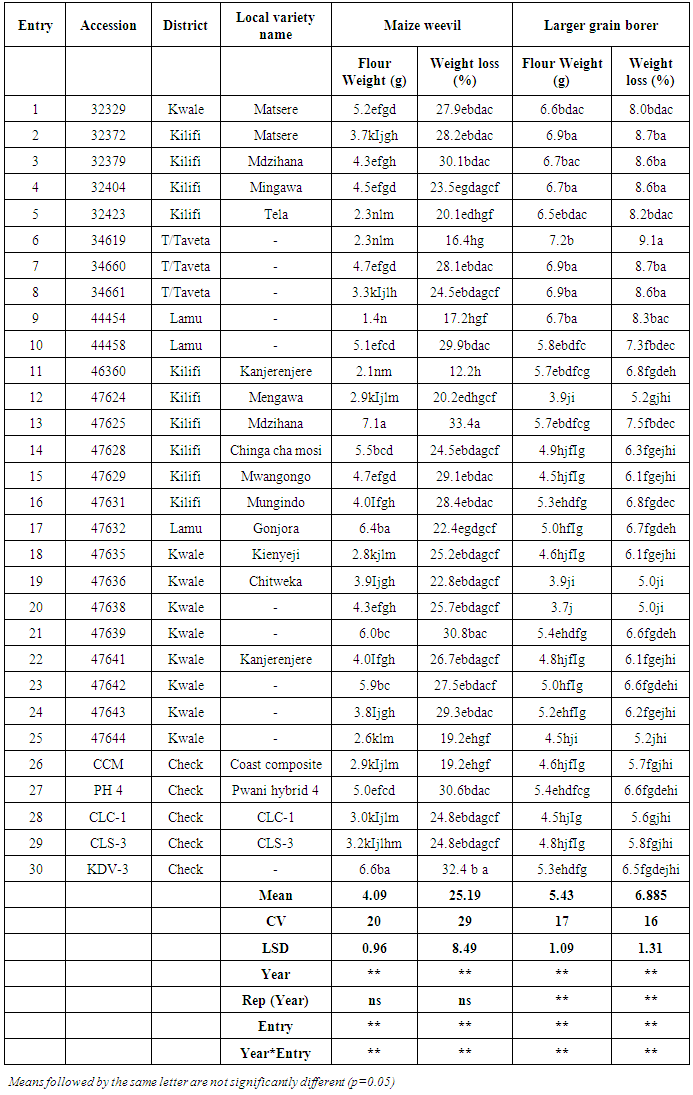
- Results of maize weevil screeningThere was a significant flour weight and weight loss caused by storage pests (Table 1). The mean flour weight of Kenyan coastal maize landraces after damage by Sitophilus zeamais, indicate that entry 9 (accession 044454) from Lamu had the lowest mean flour weight (1.4 g). Lower flour weight indicated less damage by maize weevil. Entries 2 (032372) - Matsere, 5 (0.32423) - Tela, 11 (046360) –Kanjerenjere and 12 (047624) – Mengawa, from Kilifi, 6 (034619) from Taita Taveta, 18 (047635) - Kienyeji, 24 (047643), and 25 (047644), all from Kwale were as good as the better checks, which had a mean of 3.0 g and excluded PH4 and KDV-3. Pwani Hybrid 4 (PH 4) and Katumani drought variety 3 (KDV-3) checks performed poorly with flour weights 5.0 g and 6.6 g, respectively. Entry 11 (046360) –Kanjerenjere had the lowest weight loss 12.2 %. Lower weight loss indicated less damage by weevils. Entries 4, 5, 6, 8, 9, 12, 14, 17, 18 19, 20, 22, 23, and 25 with weight losses of 16.4 – 27.5% were as good as Coast Composite, which was the best amongst the checks with 19.2% weight loss.
|
|
|
4. Conclusions
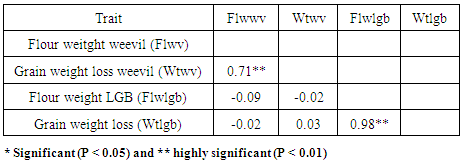
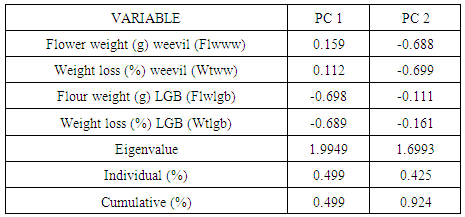
- There were resistant landraces from all districts to maize weevil. This observation is probably expected as all collections have had exposure to maize weevil for a long time as opposed to the LGB that appeared only recently. The susceptibility may partly explain why farmers grow local cultivars since PH 4 is the most readily available commercial cultivar. It appears that weevils cause more damage than LGB. From the comparisons for weight losses caused by both pests, maize weevil caused 12.2% (Kanjerenjere) - 32.4% (KDV-3) weight losses compared to only 5.0% (Chitweka) - 8.7% (Matsere) caused by the LGB. This implies that, given the same conditions, weevils are more disastrous and priority should be given to controlling them. There was variability for resistance to maize weevil and LGB in storage among Kenyan coastal maize landraces. A pattern was identified whereby, landraces from Kwale, Kilifi and Lamu showed resistance to LGB. No such pattern was observed for the maize weevil where resistance was wide spread in the region. The length of exposure to maize weevil has been considerably longer than LGB, which was observed only in the early 1990s. Landraces with superior responses to storage pests were identified, and these may be used directly or as sources of resistance in various insect resistance breeding program objectives in coastal Kenya.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML