-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2016; 6(3): 64-87
doi:10.5923/j.plant.20160603.03

Effect of Gibberellic Acid, Paclobutrazol and Zinc on Growth, Physiological Attributes and the Antioxidant Defense System of Soybean (Glycine max) under Salinity Stress
Mahmoud R. Sofy
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt
Correspondence to: Mahmoud R. Sofy, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

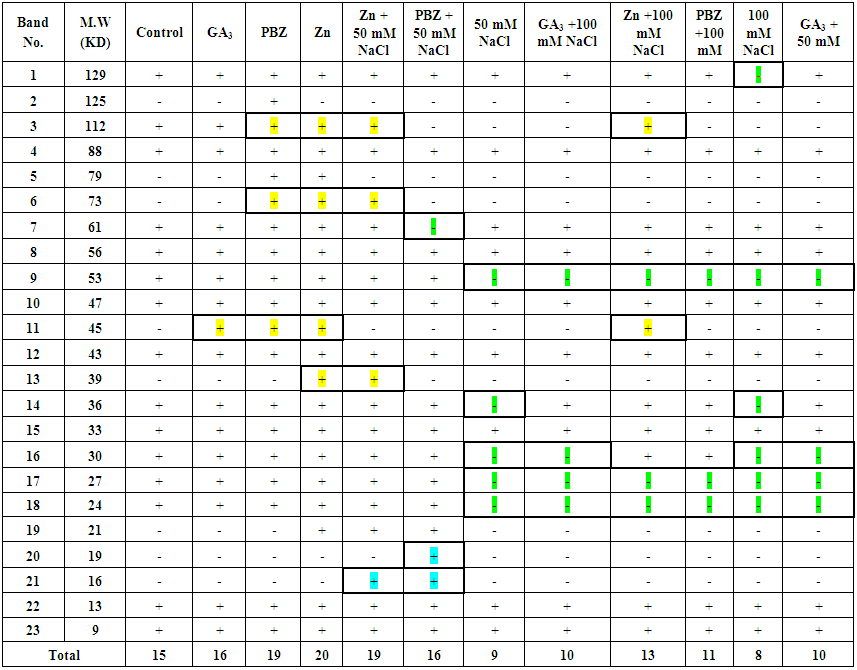
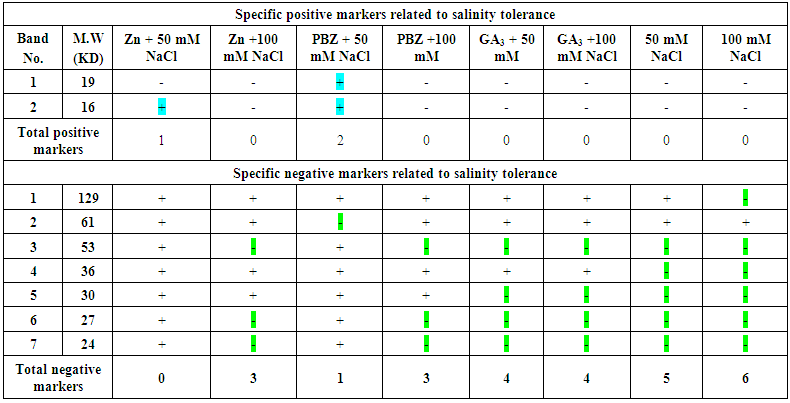
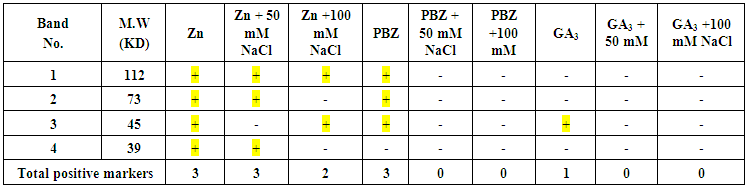
Soil salinity is one of the major abiotic stresses which caused significant reduction in the growth parameters, photosynthetic pigments and yield components of soybean plants. The present study aims to improvement of soybean production under saline conditions and also tries to elucidate the possible mechanisms of plant tolerance by using three different treatments (Gibberellic acid, Paclobutrazol and Zinc sulphate). The magnitude of reduction, increased by increasing salinity level. Application of the above mentioned treatments, in the absence and presence of NaCl, has greater changes in most of the assayed parameters where the adverse effects of salinity as regards the growth characters, photosynthetic pigments and yield components as well as soluble carbohydrates, soluble protein and oil contents in the yielded seeds were significantly mitigated by treatment with either GA3, PBZ or Zn. Salinity caused significant decreases in the activities of endogenous gibberellic acid (GA3) and indole acetic acid (IAA) while activities of both Jasmonic acid (JA) and Abscisic acid (ABA) were increased. Significant increases were observed in the activities of superoxide dismutase (SOD), peroxidase (POX), catalase (CAT) and glutathione reductase (GR) in shoots of salt stressed plants. Application of GA3, PBZ or Zn caused great variations in the activities of endogenous phytohormones and antioxidant enzymes. Treatment with either GA3, PBZ or Zn caused significant reduction in both lipid peroxidation and proline that observed in shoots and root of salinized soybean plants. The electrophoregram of protein pattern of the yielded seeds of the soybean in response to their pre-emergence treatment with different concentrations of NaCl (50 mM & 100 mM) with or without GA3, PBZ or Zn appeared variations in the number of electrophoretic protein bands. A total of 23 bands was detected with different molecular weights ranging from 129 kDa to 9 KDa. Two newly formed protein bands have appeared, 1 of them (16 KDa) at 50 mM with Zn or PBZ and the other band (19 KDa) has appeared at 50 mM NaCl with PBZ. Generally, it could be concluded that application of either GA3, PBZ or Zn have (to more extent) a beneficial regulatory role in plants grown under salt stress conditions.
Keywords: Gibberellic acid, Paclobutrazol, Zinc, Antioxidant defense system, Soybean (Glycinemax), Salinity stress
Cite this paper: Mahmoud R. Sofy, Effect of Gibberellic Acid, Paclobutrazol and Zinc on Growth, Physiological Attributes and the Antioxidant Defense System of Soybean (Glycine max) under Salinity Stress, International Journal of Plant Research, Vol. 6 No. 3, 2016, pp. 64-87. doi: 10.5923/j.plant.20160603.03.
Article Outline
1. Introduction
- Worldwide, 20% of total cultivated and 33% of irrigated agricultural lands are exacerbated by high salinity. Phenomena like low precipitation, high surface evaporation, irrigation with saline water, weathering of native rocks, and poor agricultural practices have increased the rate of soil salinization to 10% per annum. It has been predicted that more than 50% of the arable land would be salinized by the year 2050 [1]. High salt levels generate a two-component stress on plants: an osmotic stress caused by reducing water availability in soil and an ionic stress due to imbalance of solutes in the cytosol [2]. Recent research has identified various adaptive responses to salinity stress at molecular, cellular, metabolic, and physiological levels, although mechanisms underlying salinity tolerance are far from being completely understood. This paper provides a comprehensive review of major research advances on biochemical, physiological, and molecular mechanisms regulating plant adaptation and tolerance to salinity stress [3].Soybean (Glycine max L.) a legume species native to East Asia, is now widely grown as the primary oilseed crop in the world including in many regions in the world. It is noteworthy that China is currently the largest importing country for soybean despite being one of its origins. To meet the increasing demand for food, oil and protein resources, further increases in soybean production are essential [4].When plants are exposed to salinity stress, reactive oxygen species (ROS) hydrogen peroxide (H2O2), (superoxide radicals (O2–), singlet oxygen (O–) and hydroxyl radicals (OH-) are generated in response to stress conditions. ROS can cause oxidative damage to many cellular components, including membrane proteins, lipids, chlorophyll and nucleic acids [5].ROS are well- termed second messengers of cellular processes, including ability to tolerate environmental stresses [6, 7]. Whether ROS will act as signalling molecule or damaging depends on the delicate equilibrium between scavenging and ROS production. Due to the multifunctional roles of ROS, it is essential for the cells to switch the level of ROS tightly to prevent any oxidative injury. Detoxing of excess ROS or scavenging is achieved by an efficient antioxidative system comprising of the enzymic in addition to nonenzymic antioxidants [8]. The enzymic antioxidants include superoxide dismutase (SOD) is a major scavenger of O2– and its enzymatic action results in the production of H2O2 and O2. Then, H2O2 is scavenged by a variety of peroxidases (POX) or directly broken into water and oxygen by catalases (CAT), glutathione reductase (GR). Glutathione reductase activity regulates the redox potential of cells and is it play an important role in the physiological under oxidative stress. The role of GR is to protect the cell against oxidative stress effects by maintaining a high reduced glutathione membrane to-oxidized glutathione (GSH/GSSG) ratio [9, 10].In situations of limited soil, water around the roots, some compounds such as proline accumulate within the cells to supply appropriate conditions for absorbing water. Proline, that's usually regarded as an osmoprotectant, is proven to be involved in tolerance mechanisms against oxidative stress, the main strategy that plants use to avoid harmful results of abiotic stresses [11].Exogenous application of different plant growth regulators is a well-recognized strategy to alleviate stress-induced adverse effects on different crop plants by regulating a variety of physiobiochemical processes such as photosynthesis, chlorophyll biosynthesis, nutrient uptake, antioxidant metabolism, and protein synthesis, which are directly or indirectly involved in the mechanism of stress tolerance [12]. The incorporation of plant growth regulators (PGRs) during presoaking treatments in many crops has improved seed performance under saline conditions [13]. It is also possible that under highly saline conditions, naturally present hormones and seed soaking with plant growth regulators helps to ameliorate the adverse effects of salinity by supplying hormones for normal growth. Phytohormones are known to influence a number of physiological processes, including enzyme activation. One of the most effective ways to overcome the problems of salinity is the use of plant growth regulators. Chauhan et al. [14] suggested that plant growth regulators help in overcoming the harmful effects of salinity on growth by changing the endogenous growth regulators which affect plant water balance.This study was designed to investigate the effect of either GA3 (as growth promoter), PBZ (as growth retardant) or Zn (as micronutrient), NaCl and their interactions on growth, yield and some metabolic activities in soybean plants hopping to elucidate the role of these substances in alleviating the adverse effects of salt stress.
2. Materials and Methods
2.1. Methods of Planting, Treatments and Collection of Samples
- The seeds of soybean (Glycine max L. var. klark) were obtained from the Agricultural Research Centre, Ministry of Agriculture, Giza, Egypt. A pot experiment was carried out in Botanical farm, Fac. of Sci., Al-Azhar Univ., Cairo, Egypt. Seeds were sown in pots 45 cm diameter. Each pot was filled with 10 kg of clay loamy soil (40% clay, 35% silt & 25% sand) with 15 seeds/pot. Thinning was performed after 1 week later of germination leaving Ten plants per pot. The pots were divided into four sets representing the following; first set contains (control, 50 mM NaCl, & 100 mM NaCl), the second set contains (GA3, GA3 + 50 mM NaCl & GA3 + 100 mM NaCl), the third set (PBZ, PBZ + 50 mM NaCl, PBZ + 100 mM NaCl) and the fourth set (Zn, Zn + 50 mM NaCl, Zn + 100 mM NaCl). The seeds and the developed seedlings irrigated with fresh water (control), 50 mM NaCl and 100 mM NaCl. While, each of GA3 (200 ppm), PBZ (200 ppm) and Zn (150 ppm of ZnSO4) was applied as presoaking (two hours) and as foliar twice when plants at 48 and 72 days old. Samples were collected for analysis when the plants 58 (Stage I) and 82 (Stage II) days old. At the end of the growth season analysis of the yield as well as the yielded seeds from the different treatments and controls were done.
2.2. Phytochemical Contents
- Photosynthetic pigments and carotenoids were estimated using the method of Vernon and Selly [15]. Contents of soluble protein of seeds were estimated according to the methods of Lowery et al. [16]. Contents of soluble carbohydrate of seeds were measured according to the method of Umbriet et al. [17]. The oil content of seed was determined according to the method of AOAC [18] using soxhelt apparatus and petroleum ether (40-60ᴏC) as a solvent.
2.3. Assay of Enzymes Activities
- Protein enzymes were extracted according to the method of Kherjee and Choudhuri [19]. Super oxide dismutase (SOD) activity was measured according to the method of Dhindsa et al. [20]. Peroxidase (POX) activity was assayed using the method of Bergmeyer [21]. Catalase (CAT) activity was assayed according to the method of Chen et al. [22]. Glutathione reductase (GR) activity was assayed according to the method of Karni et al. [23].
2.4. Endogenous Phytohormones
- Levels of endogenous gibberellic acid (GA3), Jasmonic acid (JA), indole acetic acid (IAA) and abscisic acid (ABA) were determined for all treatments and the controls using HPLC according to the method of Lee et al. [24].
2.5. Determination of Lipid Peroxidation
- Lipid peroxidation was determined by estimating the malondialdehyde (MDA) content according to Herna´ndez and Almansa [25], fresh weight samples (500 mg) were homogenized in 5 ml of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000g for 20 min at 4°C. One ml aliquot of the supernatant was mixed with 3 ml of 0.5% thiobarbituric acid (TBA) prepared in 20% TCA and incubated at 90°C for 20 min. After stopping the reaction in an ice bath, samples were centrifuged at 10,000g for 5 min. The supernatant absorbance at 532 nm was then measured.
2.6. Determination of Proline Content
- For determination of the proline content, shoot and roots were hand-homogenized in 3% of sulfosalicylic acid and centrifuged at 3000g at 4°C for 10 min. The supernatants were used for proline estimation according to the method of Bates et al. [26].
2.7. Electrophoreses
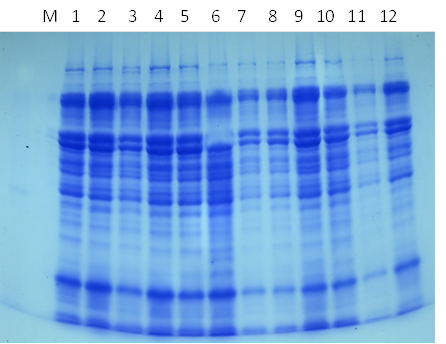
- Total soluble proteins of seeds were analyzed using sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. Soybean seeds (0.5 g) were homogenized with 2 ml of a buffer containing 50 mM Tris (hydroxymethyl) aminomethane (Tris)-Glycine (pH 8.3), 0.5 ml sucrose, 50 mM EDTA, 0.1 ml KCl, 2 mM PMSF and 0.1% (v/v) 2-mercaptoethanol in a chilled pestle and mortar at 4°C. The homogenate was centrifuged in a refrigerated centrifuge (Sigma, 2K15, Germany) at 14,000g for 10 min. Protein concentration in the supernatant samples was estimated according to the method of Lowery et al. [16]. The supernatants were stored in small aliquots at -40°C for SDS-PAGE.Supernatant samples (40 μg protein) were mixed with equal volumes of solubilizing buffer [62.5 mM Tris-HCl, pH 6.8, 20% (w/v) glycerol, 2% (w/v) SDS, 5% (v/v) 2-mercaptoethanol and 0.01% bromophenol blue] and heated for 4 min at 95°C, and then it cooled on ice. Polypeptide pattern was analyzed on 12% SDS polyacrylamide gels according to the method of Laemmli [27], as modified by Studier [28]. The gels were stained with 0.25% Coomassie Brilliant Blue R-250 (Sigma) in 50% (v/v) methanol and 10% (v/v) acetic acid for 2 h and destained with 50% (v/v) methanol and 10% (v/v) acetic acid until the background was clear. The gels were photographed and scanned using a densitometer (GS- 710, Bio-Rad, USA) and analyzed with AlphaEaseFCTM ver. 4 software.
2.8. Statistical Analysis
- We calculate sample size according to Raosoft, and all statistical calculations were done using SPSS (statistical package for the social science version 20.00) statistical program at 0.05 level of probability [29]. Quantitative data with parametric distribution were done using Analysis of variance the One-way ANOVA and Post hoc-LSD tests (the least significant difference). The confidence interval was set to 95% and the margin of error accepted was set to 5%. The p-value was considered non-significant (NS) at the level of > 0.05, significant at the level of <0.05, 0.01 and highly significant at the level of < 0.001. Discriminant analysis, automatic and linear modelling were estimated to show the relationship between quantitative parameters [30].
3. Results and Discussion
3.1. Growth Characters
- The obtained results (Figs. 1-7) showed a retarded growth in salt-stressed plants. Shoot length, root length, fresh and dry weights of both shoots and roots and the number of leaves/plant were significantly decreased under saline condition. Decreases in the aforementioned characters were increased with increasing the level of salinity. Many studies have shown that biomass partitioning between roots and shoots is strongly influenced by the most limiting resource under stress growth conditions, and resource deficiency is often ameliorated by increasing the biomass allocation to the part of the plant responsible for acquiring the most limiting resource [31, 32]. The reduction in root and shoot development may be due to the toxic effects of the NaCl used as well as unbalanced nutrient uptake by the plants competitors between Na+ and Cl- and further anions and cations may result in a reduced plant growth and yield [33, 34].Results of the present study (Figs. 1-7) revealed that, with a few exceptions, the growth characters of salinized and non-salinized soybean plants were increased under the application of each of GA3, PBZ and Zn. The aforementioned increases, in most cases, were found to be statistically significant. These results are more obvious in plants grown at the first level of NaCl (50 mM). The adverse effects of salinity as regards the growth parameters were significantly alleviated by the application of growth regulators [35]. In this regard, the applications of gibberellins increase the seed germination percentage by attributing the fact that they increase the amino acid content in embryo and cause release of hydrolytic enzyme required for digestion of endospermic starch when seeds renew growth at germination. Gibberellic acid acts synergistically with auxins, cytokinins and probably with the other hormone, is what might be called a system approach, or synergism. The overall development of the plant is regulated by the growth hormones, nutrient and environmental factors [14]. Regarding the treatment with PBZ, Hajihashemi and Kiarostami [36] suggest that PBZ treatment may be useful to improve the salt tolerance of wheat via reducing the negative effect of salinity on vegetative growth. Also, Mahmoud [37] revealed that, under non saline conditions, treatments with paclobutrazol (75 ppm) generally enhanced most of the growth and yield characteristics represented by the length of shoot & root length, fresh and dry weights of shoots and roots/plant and seed index of soybean plants. Regarding the treatment with Zn, some investigations illustrate the potent effect of Zn in overcoming the adverse effects of salinity on plant growth and development. In this concern, Anita et al. [38] recorded significant values of growth and yield of Vigna radiata plants grown under saline conditions in response to treatment with Zn. Moreover, Aldinary [39] revealed that, under saline conditions 4000 & 8000 ppm NaCl, treatments with Zn (100 ppm), in cowpea plants, generally enhanced most of the growth and yield characteristics (shoot length, root length, fresh and dry weights of shoots and roots/ plant 100 seed weights).
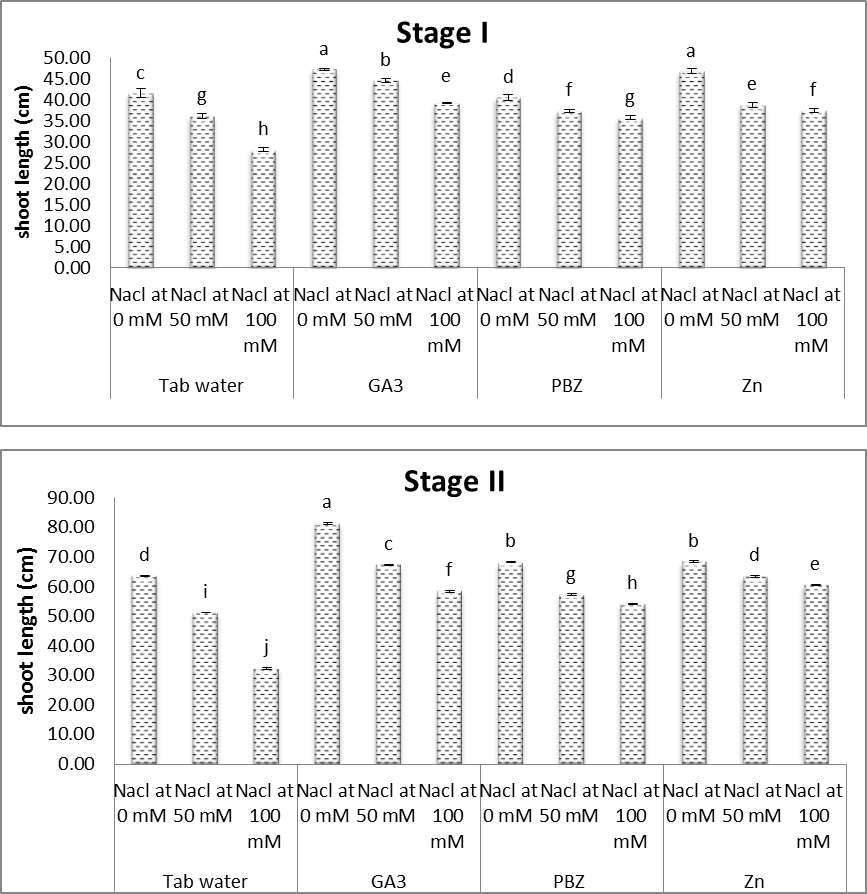 | Figure (1). Effect of salinity, GA3, PBZ, Zn and their interactions on shoot length of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
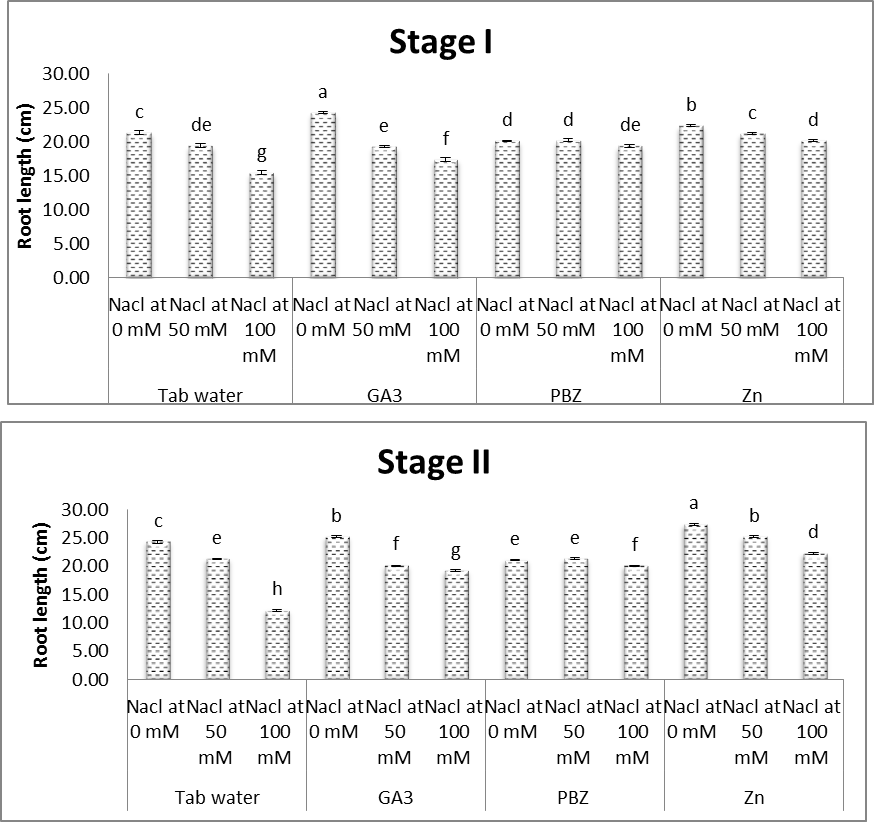 | Figure (2). Effect of salinity, GA3, PBZ, Zn and their interactions on root length of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
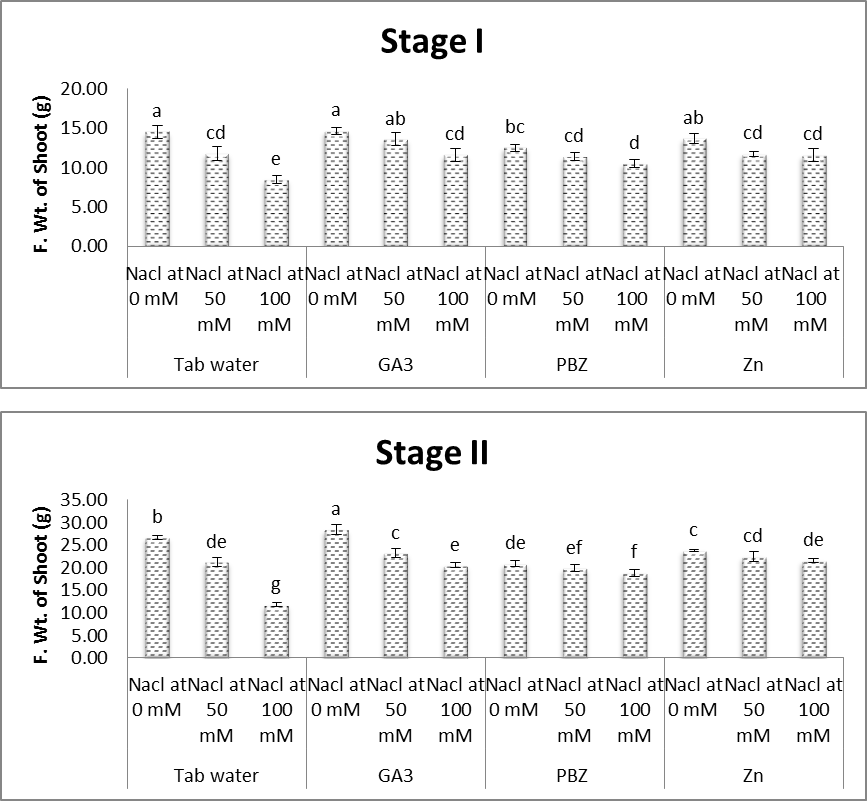 | Figure (3). Effect of salinity, GA3, PBZ, Zn and their interactions on fresh weight shoot of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
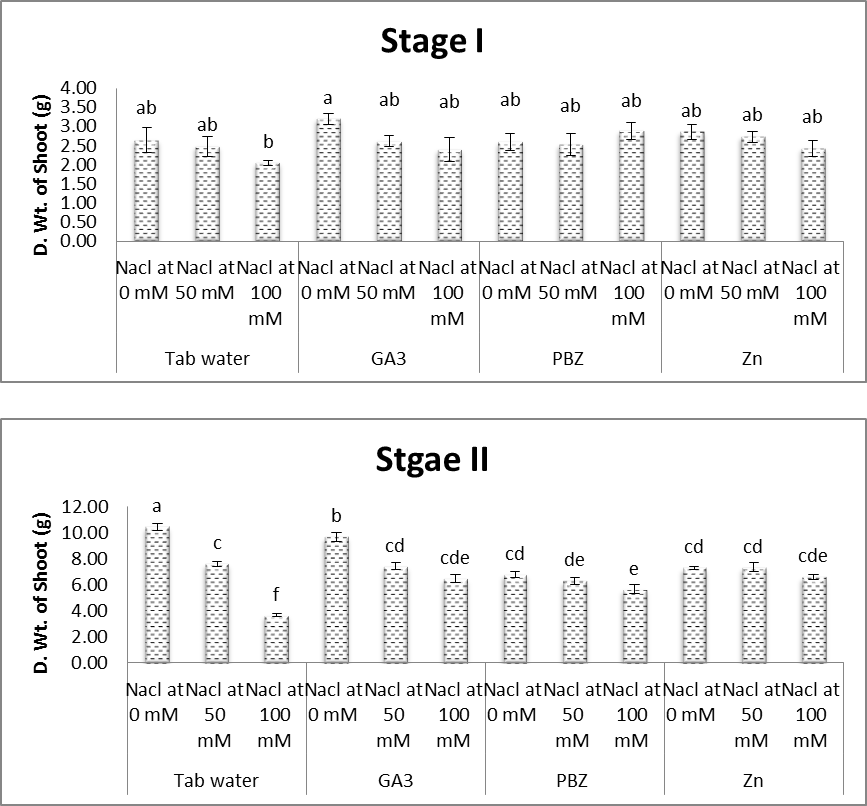 | Figure (4). Effect of salinity, GA3, PBZ, Zn and their interactions on dry weight shoot of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
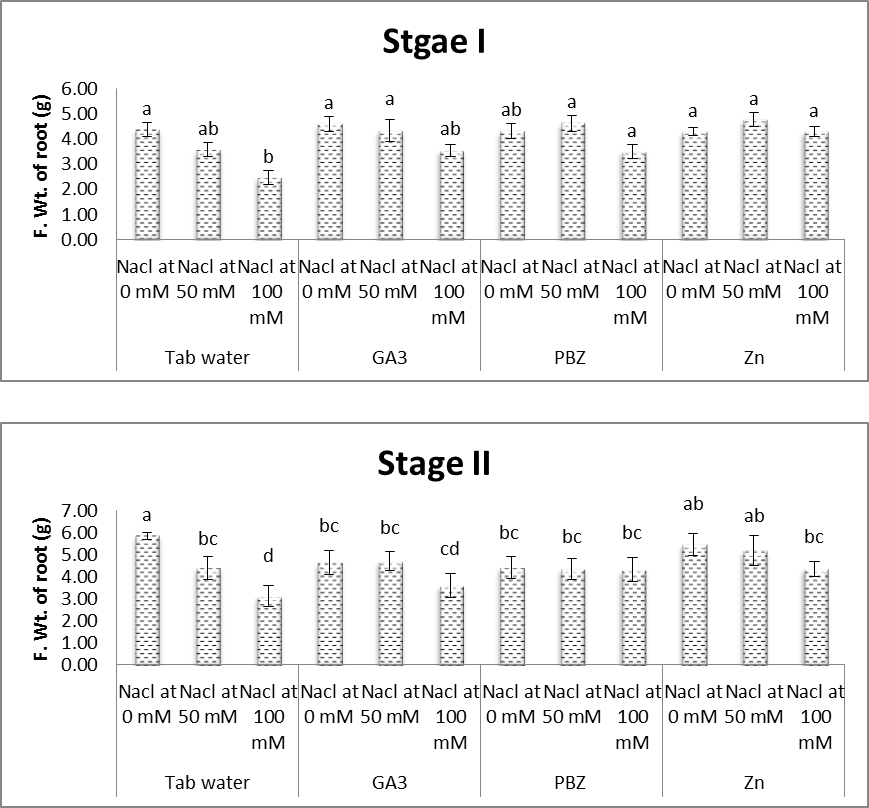 | Figure (5). Effect of salinity, GA3, PBZ, Zn and their interactions on fresh weight root of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
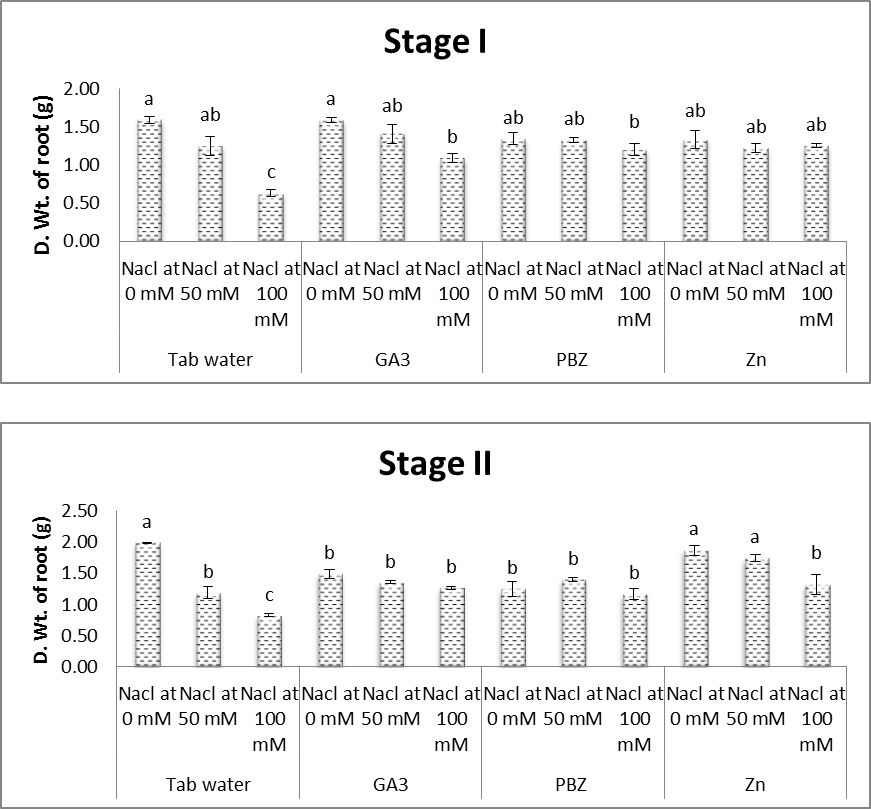 | Figure (6). Effect of salinity, GA3, PBZ, Zn and their interactions on dry weight root of soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
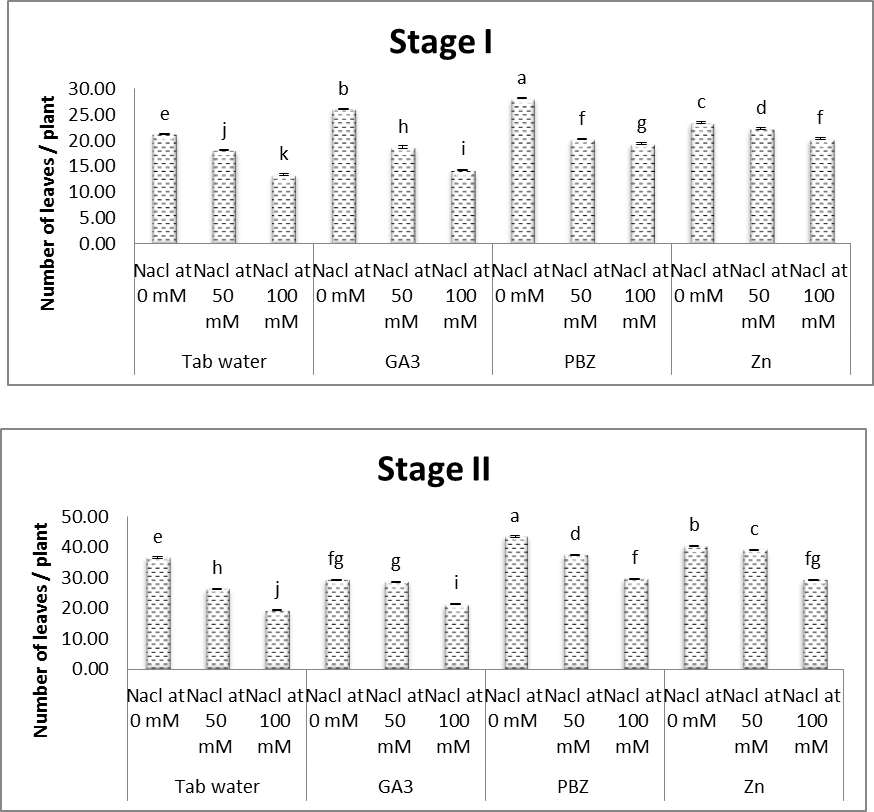 | Figure (7). Effect of salinity, GA3, PBZ, Zn and their interactions on number of leaves/soybean plants. The bars with the same letters are not significantly different at P ≥ 0.05 |
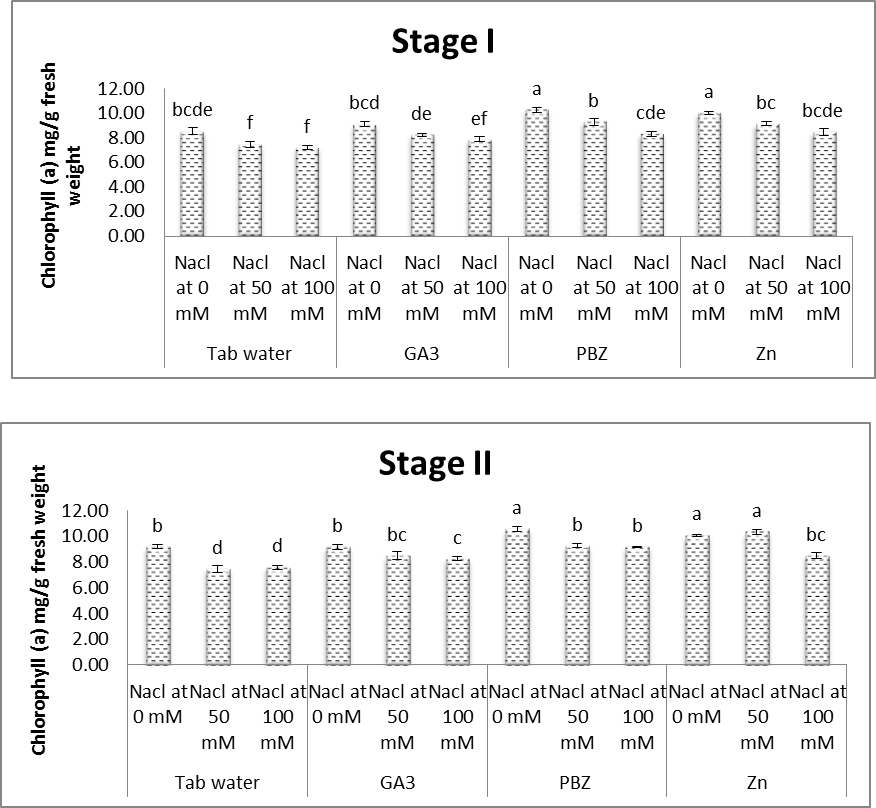
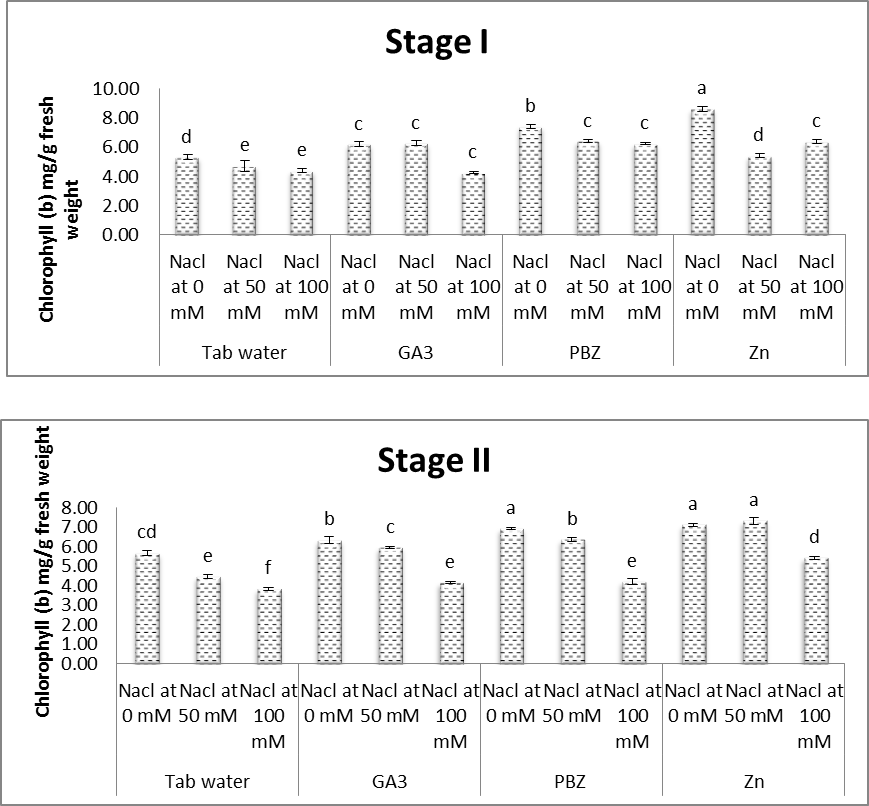
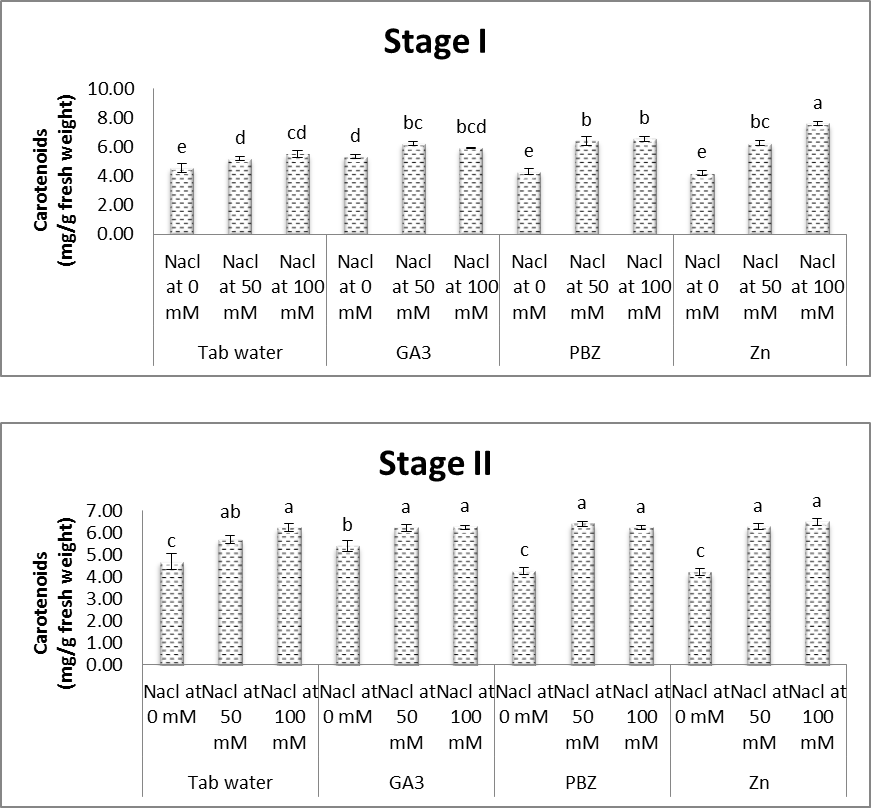
3.2. Photosynthetic Pigments
- As shown in figures (8 & 9), contents of chlorophyll a & b in leaves of soybean plants were significantly decreased in response to saline conditions. The decrease in chlorophyll contents was increased with increasing salinity level. At the same time, the obtained results (Fig. 10) showed that carotenoid content in leaves of soybean plants were significantly increased under salt stress conditions. Several investigators confirmed that salinity adversely affects the photosynthetic pigments of different plants (Almodares et al. [40] on sorghum, Abeer [41] on Vigna sinensis and Aldinary [39] on cowpea plants. Mane et al. [42] revealed that decrease in chlorophyll content under NaCl salinity to the disruption in cellular functions and membrane deterioration. Disrupted photosynthetic electron transport chain or stability of the pigment protein complex with increased activity of chlorophyllase may also be the reason for the decrease in chlorophyll content under saline conditions.
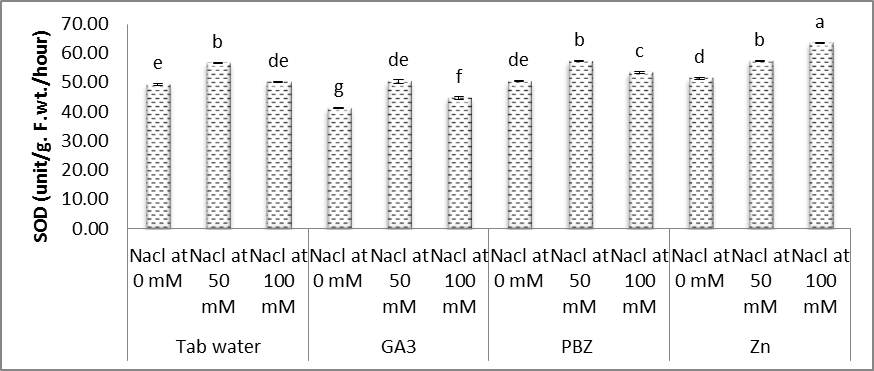
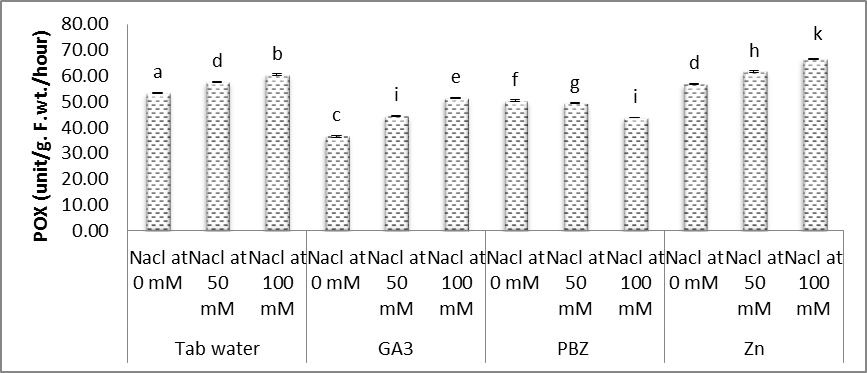
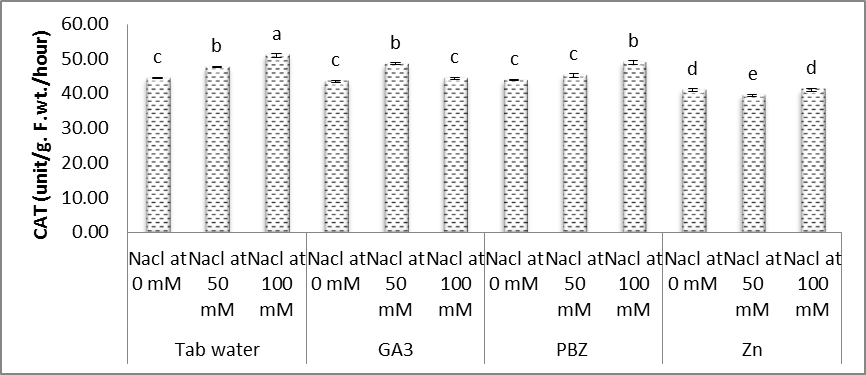
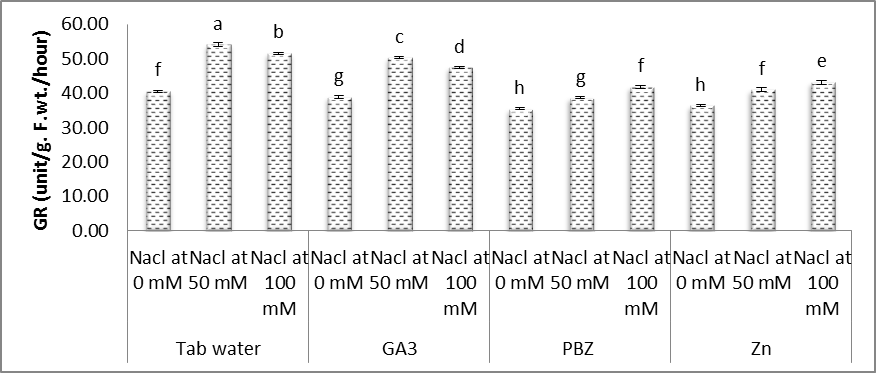
3.3. Antioxidant Enzymes
- In the present study (Figs. 11-14), significant increases were observed in the activities of SOD, POX, CAT and GR in the shoots of soybean plants under salt stress conditions. Moreover, it was found that the activities of antioxidant enzymes were increased with increasing salinity level. Even under optimal conditions, many metabolic processes produce ROS. The production of toxic oxygen derivatives is increased as a result of all types of abiotic or biotic stresses. Plants possess efficient systems for scavenging active oxygen species that protect them from destructive oxidative reactions [45].As part of this system, antioxidant enzymes are key elements in the defense mechanisms. Changes in the activities of the antioxidant enzymes under saline conditions has been reported by several investigators, increased in the case of salt-tolerant cotton [46], shoot cultures of rice [47], cucumber [48] and wheat [32]. In this respect, the effects of salt stress on the antioxidant enzymes are very complex and depend on the treatment time, plant species and genotypes [49]. Shen et al. [50] found that the activities of catalase (CAT), peroxidase (POX), superoxide dismutase (SOD) and hydrogen peroxide were increased under stress conditions on soybean plants.The enzyme is involved in dismutation of superoxide radicals to hydrogen peroxide and oxygen [51]. In the present study, SOD activity has increased with increasing NaCl level concentration and a treatment period (Fig. 11). Soybean plants exhibited much more elevated SOD activity and for that reason could possess better oxygen free radicals scavenging certain capacity minimizing the oxidative damage. Published reports also showed that demonstrated that over expression of SOD led to efficient, stress protection against NaCl stress in plants such as B. maritima and B. vulgaris [52], cotton [46], maize [53].Superoxide dismutase action leads to the production of H2O2 a highly toxic molecule to living cells, which needs to be eliminated from plant cells in subsequent reactions. Under such stress conditions enzymes, for example CAT and POX are usually activated and active in the removal of H2O2 scavenging enzyme in plant leaves [53]. Our results showed that enzyme activities of CAT, POX elevated considerably under salt stress conditions over their controls. High catalase activity boosts, the cell membrane stability by decreasing H2O2 content under NaCl stress conditions [54]. We demonstrated that, rise in CAT, POX activities follows elevated SOD activity under salt stress. Our results offer the hypothesis that antioxidative enzymes play a main protective role within the detoxing of O2 and H2O2 by scavenging process with coordination of SOD [55, 56].Glutathione reductase (GR) is a vital enzyme involved with converting of facets of GSSG to GSH under ecological stress conditions [13]. Within our study, the GR activity increased with NaCl concentration, with an increased prominent raise being observed with control plants. Raise in activity under Salinity stress seemed to be proven in the past reports on cucumber [48] and barely [55].Results in figures (11-14) revealed that, under saline and normal conditions, treatment with either GA3, PBZ or Zn, in most cases markedly reduced the over increases in the activities of POX, CAT and GR, however the activities of SOD was increased than that of the corresponding controls. Similar results were obtained in the work of Aldinary [39] who found that treating cowpea plants with either PBZ or Zn increased the activities of SOD in shoots of plants grown in either saline or non-saline conditions. Tuna et al. [57] reported that POX, CAT activity in salt-stressed maize plants was decreased by exogenous application of GA3.
3.4. Endogenous Phytohormones
- Results of the present investigation (Fig. 15) showed that salinity greatly affected the activities of the endogenous phytohormones in shoots of soybean plants. Under the first level of salinity (50 mM NaCl), the content of both JA and ABA was markedly increased than that of the control ones. At the same time, the content of both GA3 and IAA was significantly decreased in response to the first level of NaCl. At the second level of NaCl (100 mM), significant decreases were observed in the content of JA, GA3 and IAA, while the content of the ABA was found to be highly significantly increased. In this regard, El-Khallal et al. [58] found that salt stress led to sharp decrease in the levels of IAA, GA3 and Zeatin, while ABA level greatly increased in maize shoots. These results appeared that salt stress led to sharp changes in the balance of endogenous hormones, which associated with the accumulation of ABA and decrease in the level of and cytokinins. Thus, reduction in shoot growth of tomato plants is probably related to hormonal signals generated in response to salt stress as suggested by Ghanem et al. [59].Results of the present study (Fig. 15) revealed that the adverse effects of salinity as regards the endogenous GA3 and IAA were markedly alleviated by treatment with either GA3, PBZ or Zn. This was the case with plants grown in either saline or non-saline conditions. On the other hand, significant decreases in the content of JA and ABA were observed in plants grown in salinized conditions due to application of GA3. Treatment with PBZ or Zn was found to be more effective than that of GA3 in deceasing the contents of endogenous JA and GA3 in salinized soybean plants. The potent effect exogenous application of GA3, PBZ or Zn as regards the activities of endogenous phytohormones was investigated by others. In this concern, Aldinary [39] found that treating cowpea plants with either PBZ or Zn markedly increased the endogenous contents of GA3 and IAA, while the content of endogenous ABA was decreased. This was the case with plants grown under saline conditions.
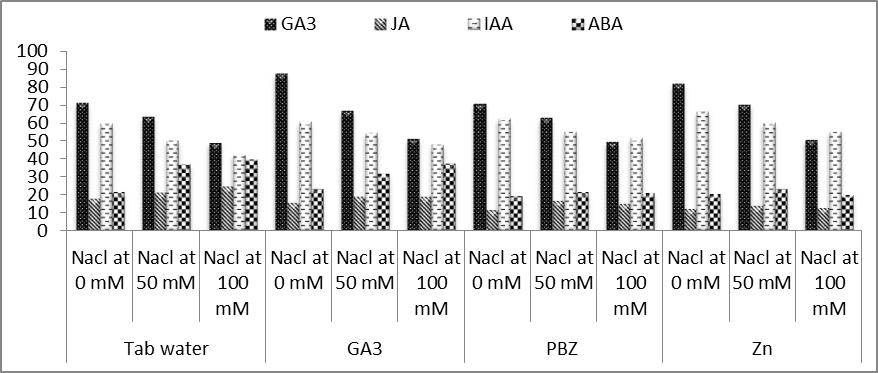 | Figure (15). Effect of salinity, GA3, PBZ, Zn and their interactions on contents of endogenous hormones of soybean plants |
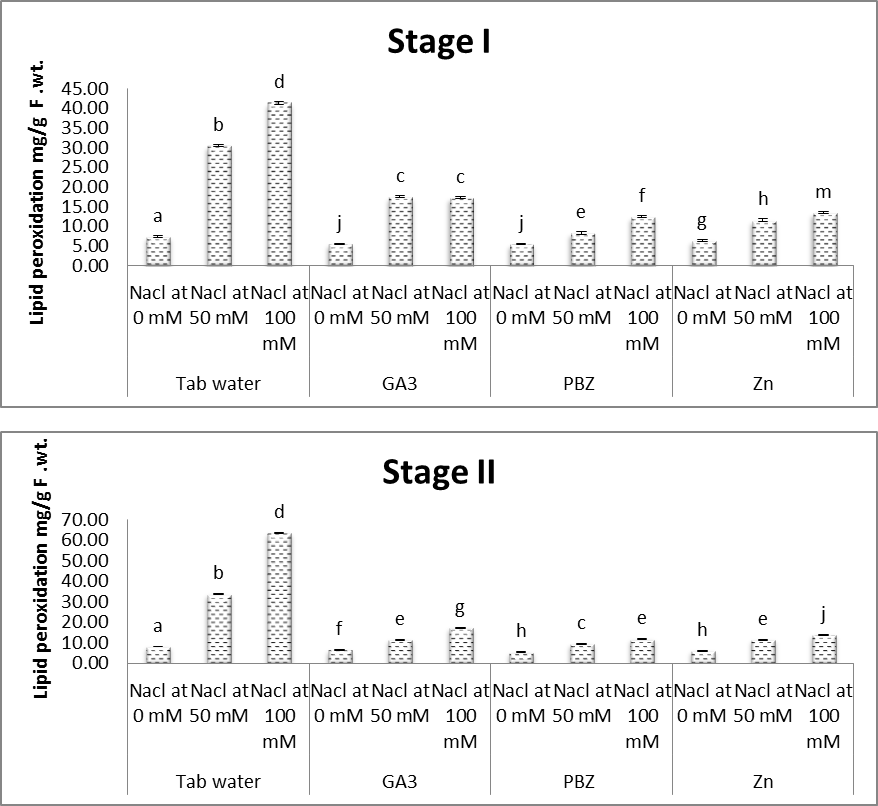
3.5. Lipid Peroxidation
- Lipid peroxidation represented by the determined content of malondialdehyde (MDA) as shown in figure (16). Significant increases were observed in the contents of MDA in the shoots. This was the case throughout the duration of the experiment and under the two applied levels of salinity. In this regard, Hulusi et al. [60] under the effect of NaCl treatment MDA content increased significantly during the experimental period in sesame cultivars as compared to control groups Naureen and Naqvi [61] measured H2O2 and MDA concentrations in salt stressed wheat plants that are oxidative stress indicators. H2O2 caused membrane damage fasten the Haber-Weiss reaction by production of hydroxyl radical led to increase lipid peroxidation. Our results are also in agreement with those of Shalata and Neumann [62] reported that salt-stress increased the accumulation of lipid peroxidation products produced by interactions with damaging active oxygen species in stems of tomato plants.Verma and Mishra [63] reported that salinity caused a reduction in seedling growth and biomass accumulation, which was parallel to that caused by increased superoxide (2O−), hydrogen peroxide (H2O2) levels, lipid peroxidation and electrolyte leakage in leaf tissues.On the other hand, the results of the present study (Fig. 16) revealed that treatment with either GA3, PBZ or Zn significantly decreased the content of MDA (lipid peroxidation) in shoots of soybean plants. It is worth to mention that treatment with PBZ or Zn was found to be more effective than GA3 in deceasing the MDA content in shoots of soybean plants. This was the case with plants grown in either saline or non-saline conditions and under the two applied levels of salinity. The inhibitory effect of sufficient concentrations of Zn application on the production of these injurious components in saline conditions has been reported by other authors [64-66]. Zinc plays a key role in controlling the generation and detoxification of free oxygen radicals and subsequent lipid membrane oxidation [67]. It has been demonstrated that Zn ions have a strong inhibitory effect on membrane bound, NADPH oxidase [68].
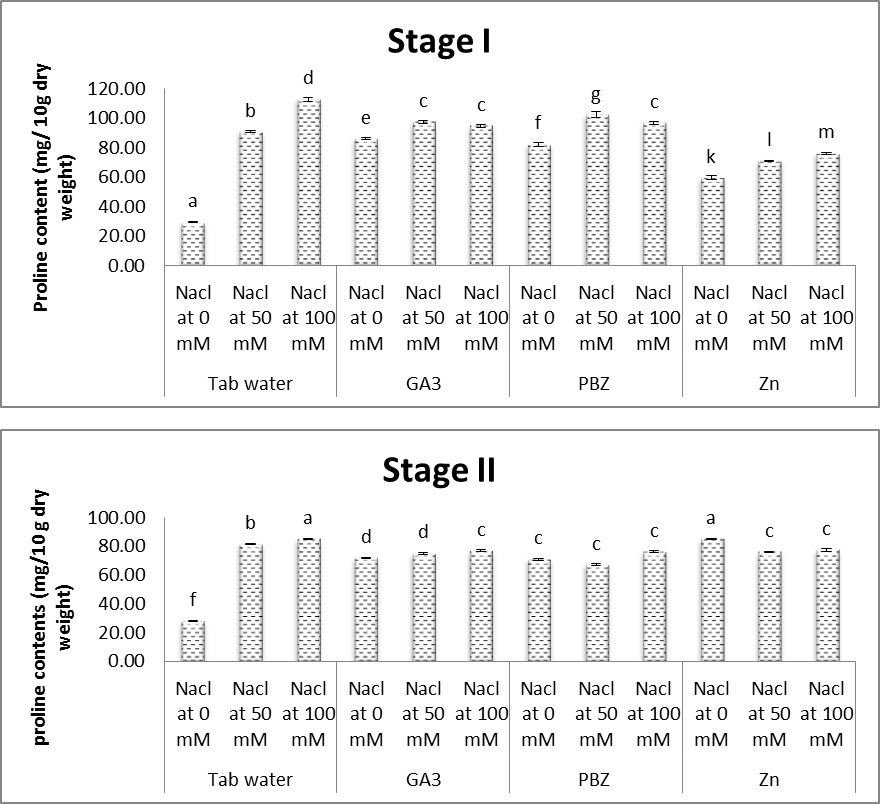
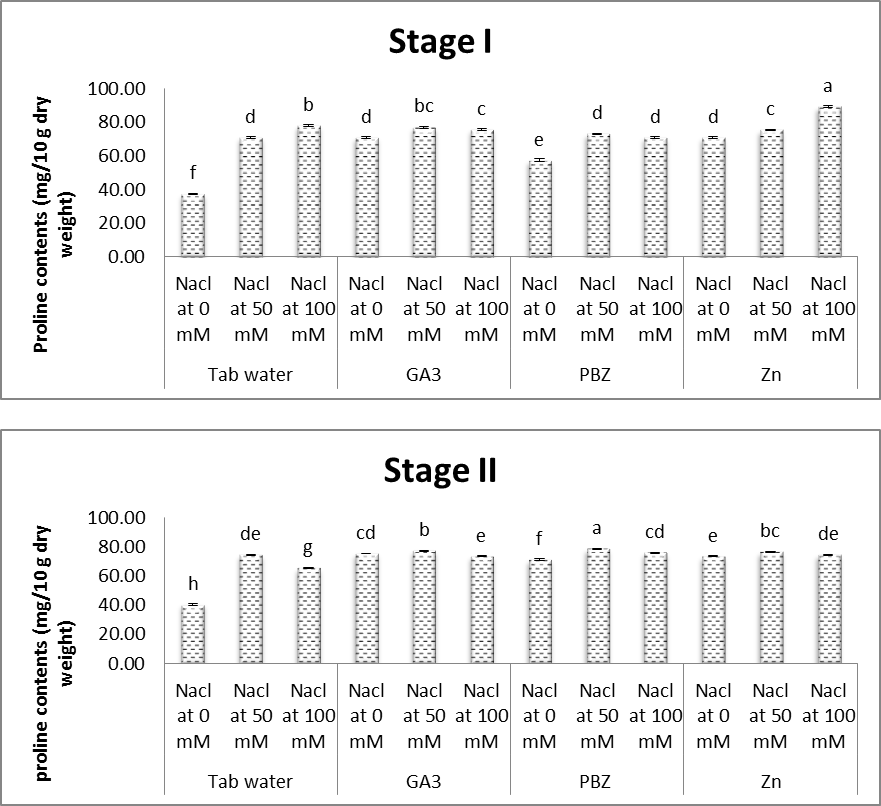
3.6. Free Proline
- The obtained results (Figs. 17 & 18) showed that, free proline content in both shoots and roots of soybean plants was significantly increased under salt stress conditions. Increases in the proline contents were more obvious with increasing the level of salinity. This was the case throughout the duration of the experiment. Ashraf and Foolad [69] reported that in organisms ranging from bacteria to higher plants, there is a strong correlation between increased cellular proline levels and the capacity to survive under salt stress. In addition to its role as an osmolyte for osmotic adjustment, proline contributes to stabilizing sub cellular structure (membrane and proteins) scavenging free radicals and buffering cellular redox potential under stress conditions. The obtained results are in agreement with Amini and Ehsanpour [70] in tomato, Radyukina et al. [71] in Geum urbanum L. and Lobato et al. [72] in Vigna unguiculata L. Accumulation of some compatible solutes (TSS, proline and free amino acids) in stressed plants produced lower solute potential, which allows plant cell to maintain a higher water content than the corresponding control. These solutes play an important role in plants under stress conditions, where major functions of sugars are osmoprotection and/or osmotic adjustment as reported by Sharma et al. [73].Results of the present study (Figs. 17 & 18) revealed that treatment with either GA3, PBZ or Zn significantly decreased the content of proline in both shoots and roots of soybean plants. It is worth to mention that treatment with PBZ or Zn was found to be more effective than GA3 in deceasing the contents proline in both shoots and roots of soybean plants. This was the case with plants grown in either saline or non-saline conditions. In this regard, contrary to our results, Aldinary [39] found that treating salinized-cowpea plants with either PBZ or Zn increased the content of proline in both shoots and roots. Anjali and Aruna [43] presented that when Trigonella foenum-graecum plants were grown under saline environment, leaf proline was found to increase by about 90 percent as compared to control. However, plant growth regulators (PGR) treated plants of Trigonella foenum-graecum exhibited significant reduction in leaf proline content as compared to untreated seeds grown under 60 mM NaCl concentration, maximum reduction being observed with GA3, followed by Kinetin and BA, although, the contents were found to be slightly higher than that of control plants.
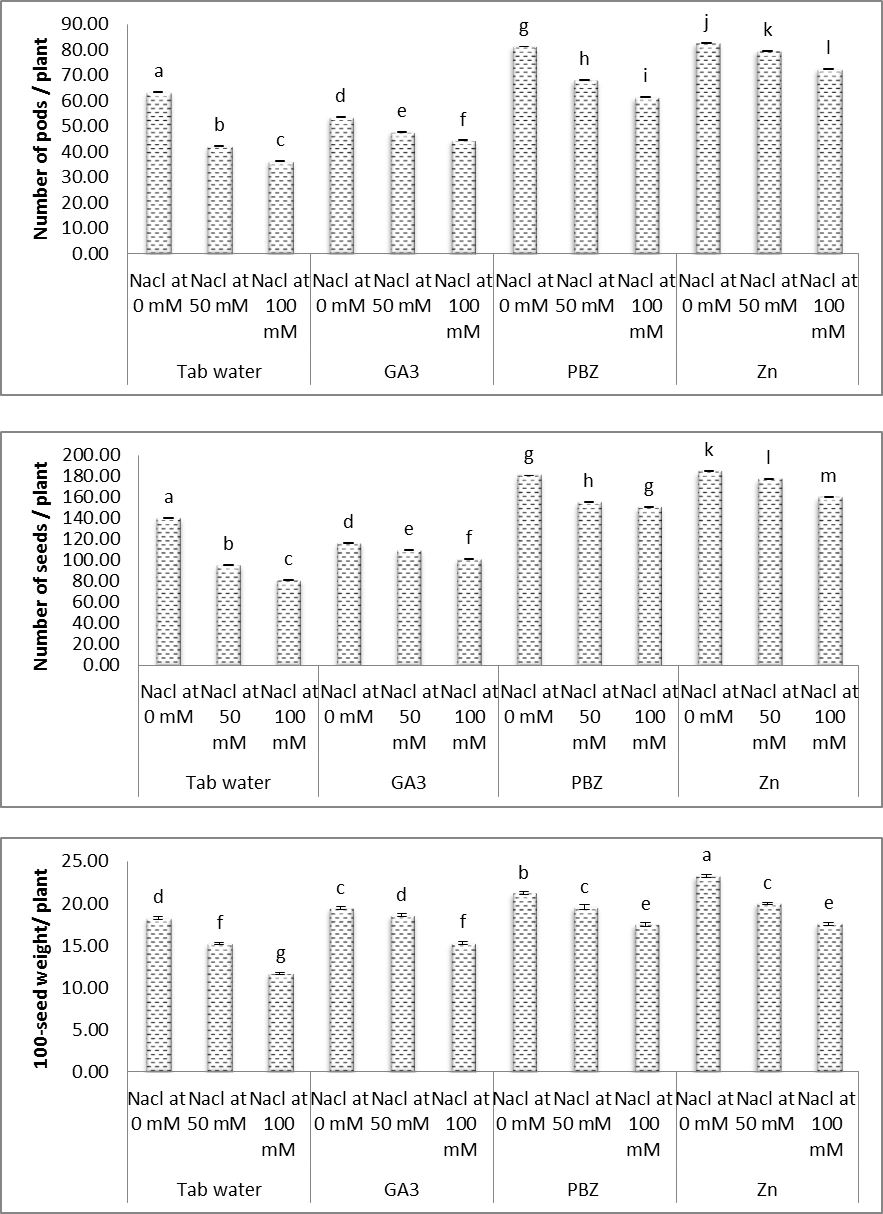
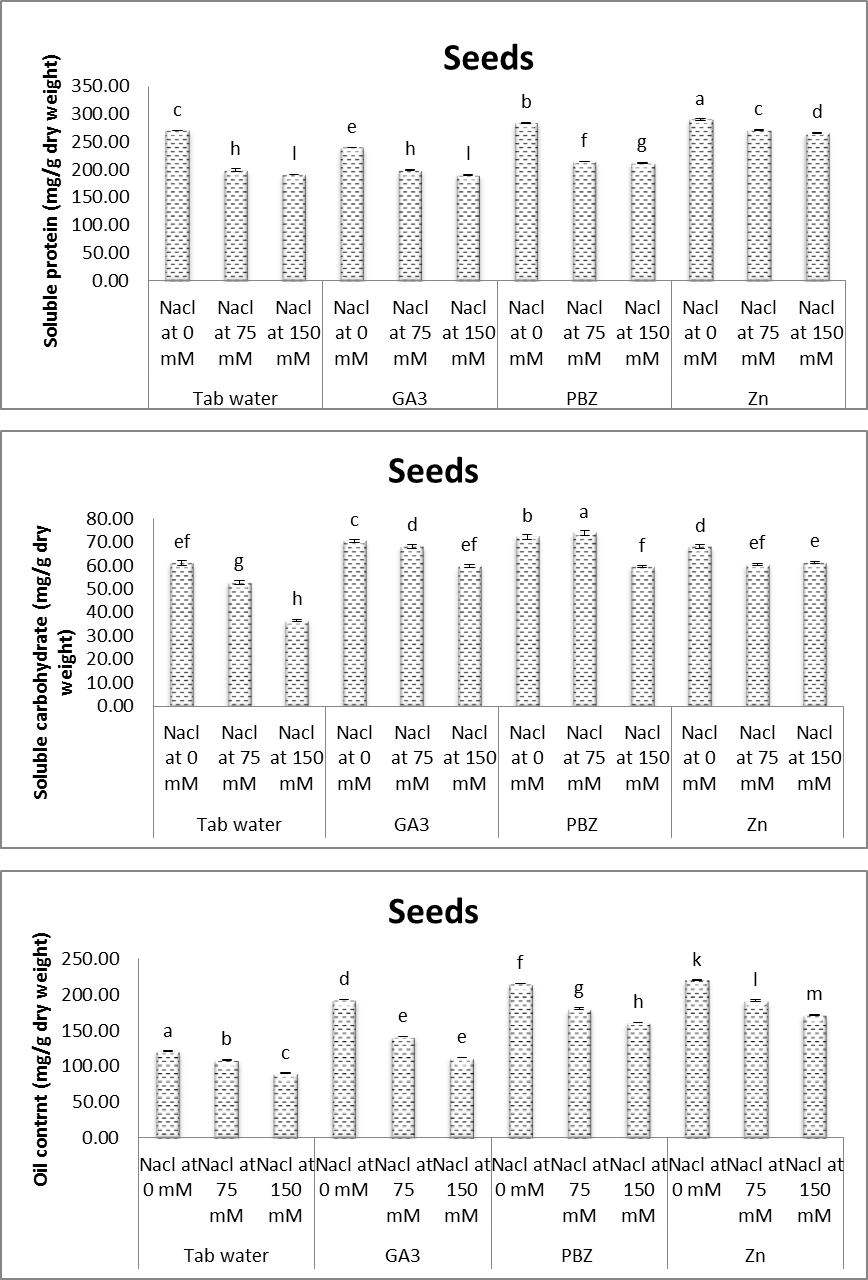
3.7. Yield and Yield Components
- Figures (19 & 20) showed the difference in including a number of pods/plant, number of seeds/plant and weight of 100 seed for each treatment as well as controls. Also, figures (19 & 20) demonstrate the content of soluble proteins, soluble carbohydrates and oil content in the yielded seeds for each treatment and controls. All the aforementioned parameter was significantly reduced under saline conditions. The magnitude of reduction, increased by increasing salinity level. These results are in accordance with other investigations. In this regard, Weria et al. [74] revealed that, in soybean plants, salinity (0, 33, 66 and 99 mM NaCl) caused a significant reduction in the number of pods/plant, number of seeds/plant and weight of seeds/plant. Sofy [75] revealed also that, treating lentil plants with either 20% or 30% sea water resulted in, significant decreases in the contents of total soluble carbohydrates, soluble protein in yielded seeds. Ayman et al. [76] abstracted that salinity caused significant reduction in the number of pods / plant, number of seeds/plant, weight of 100 seed, contents of soluble proteins and total oil content in the yielded seeds of soybean plants.
3.8. Protein Profile in the Yielded Seeds
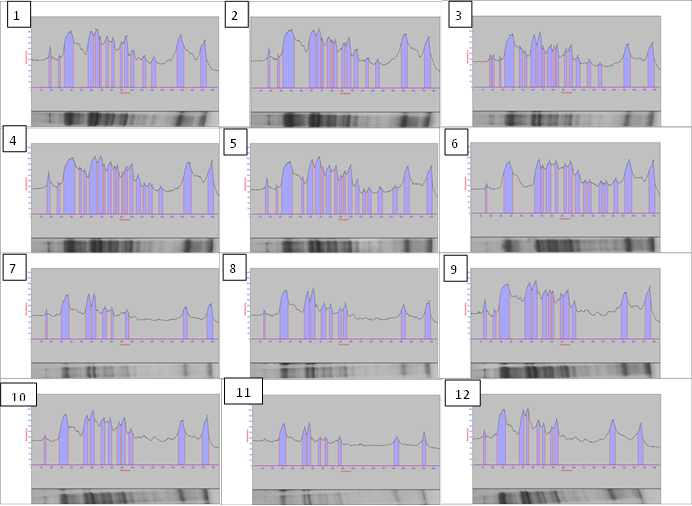
- The total soluble proteins in the yielded seeds of the soybean plant under the different treatments were separated electrophoretically using an SDS-PAGE technique in order to find out the treatments variation in specific accumulation salt induced protein upon the salt stress condition (Fig. 21 & Table 1). The major variations are expressed as changes in appearance or disappearance of some bands in the yielded seeds of the soybean in response to their pre-emergence treatment with different concentrations of NaCl (50 mM & 100 mM) with or without GA3, PBZ or Zn (Tables 1-3). A total of 23 bands were detected with different molecular weights ranging from 129 kDa to 9 KDa. These protein bands have distributed into 7 monomorphic bands (30.4%) and 16 polymorphic bands (69.6%) (Table 1).
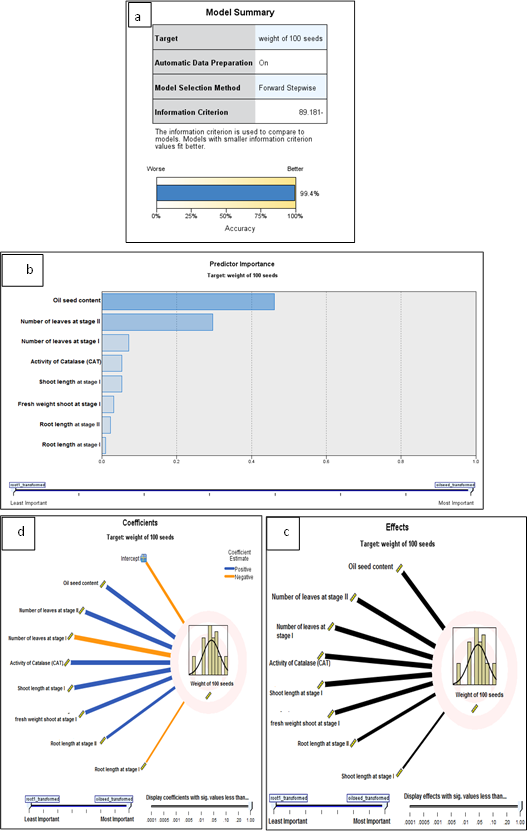
3.9. Automatic Linear Modeling and Discriminant Analysis
- Finally, when used the yielded weight as a target; we observed that the accuracy of all data about 99.4%. The effect of target weight of 100 seed, the wide line is very effective as oil content in seeds, the number of leaves at stage II, the number of leaves at stage I, activity of catalase, shoot length at stage I, fresh weight of shoot at stage I, root length at stage II, and root length at stage I. Also, the coefficient of target yield weight the positive blue color wide line is effective as oil content in seeds, and the number of leaves at stage II, activity of catalase, shoot length at stage I, fresh weight of shoot at stage I, and root length at stage II. While, negative orange color wide line is effective as the number of leaves at stage I, and root length at stage I (Fig. 23 a-d).
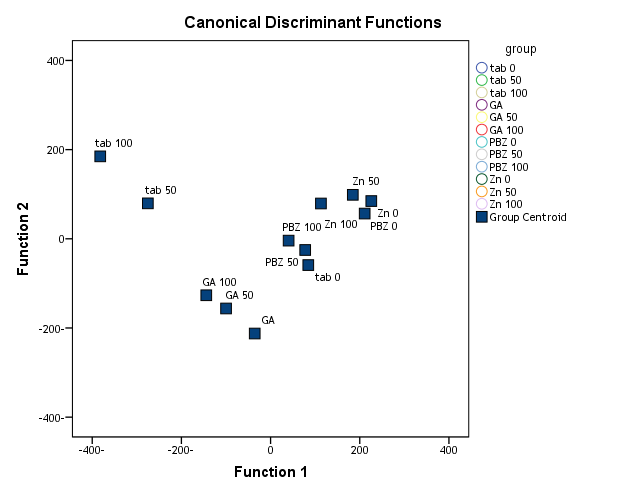 | Figure (24). Discriminant of the yielded seeds of the soybean in response to their pre-treatment with different concentrations of NaCl (50 mM & 100 mM) and or without GA3, PBZ or Zn |
4. Conclusions
- Based on present findings, it was concluded that the use of GA3, PBZ and Zn greatly improving and often maximizing the majority of the growth characteristics and yield of soybean plants grown either in saline or non-saline conditions via enhancement the majority of the physiological processes from the treated plants. It had been found in the obtained results that application of either PBZ or Zn was more efficient than GA3 in mitigating the adverse after effect of salinity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML