-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2016; 6(3): 57-63
doi:10.5923/j.plant.20160603.02

A Novel Lectin from the Fruiting Body of an Edible Mushroom Lentinus cladopus Lév.
Huidrom Rully, Sanjenbam Kunjeshwori Devi, Laishram Rupachandra Singh, Senjam Sunil Singh, Sorokhaibam Jibankumar Singh, Wayenbam Sobhachandra Singh, Helena Thongam, Hijam Kiranbala Devi
Laboratory of Protein Biochemistry, Biochemistry Department, Manipur University, Canchipur, Imphal, India
Correspondence to: Sanjenbam Kunjeshwori Devi, Laboratory of Protein Biochemistry, Biochemistry Department, Manipur University, Canchipur, Imphal, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A novel lectin was purified to electrophoretic homogeneity from the fruiting body ofan edible mushroom Lentinus cladopus Lév. by successive steps of lectin extraction, ammonium sulphate (20-60%) fractionation, discontinuous gradient Q-Sepharose chromatography, DEAE-cellulose chromatography and continuous gradient Q-Sepharose chromatography. The lectin was found to be a dimeric protein of molecular weight 40kDa made up of apparently chemically identical subunits. It exhibited a specific haemagglutinating activity of 2x105 HAU/mg protein when tested using rabbit RBC. Its haemagglutinating activity was not inhibited by any of the simple sugars, sugar derivatives, polysaccharides or glycoproteins tested. Protein mass fingerprinting by electrospray ionisation mass spectrometry and subsequent Mascot database analysis showed the purified lectin to be a novel protein, not characterised earlier.
Keywords: Mushroom, Lectin, Mushroom lectin, Lentinus cladopus, Lentinus cladopus lectin
Cite this paper: Huidrom Rully, Sanjenbam Kunjeshwori Devi, Laishram Rupachandra Singh, Senjam Sunil Singh, Sorokhaibam Jibankumar Singh, Wayenbam Sobhachandra Singh, Helena Thongam, Hijam Kiranbala Devi, A Novel Lectin from the Fruiting Body of an Edible Mushroom Lentinus cladopus Lév., International Journal of Plant Research, Vol. 6 No. 3, 2016, pp. 57-63. doi: 10.5923/j.plant.20160603.02.
Article Outline
1. Introduction
- Lectins are proteins or glycoproteins of non-immune origin which bind non-covalently and reversibly to specific carbohydrates or glycoconjugates [1]. They occur in almost all life forms and have been characterised from diverse sources including plant seeds and roots, fungi, bacteria, algae, body fluid of invertebrates, lower vertebrates and mammalian cell membranes [2]. Recently, research on lectins from different mushroom species has become an interesting field as mushrooms have long been known for their nutritive and medicinal values. Many lectins have been identified and purified from mushrooms, and their applications in medicine, therapeutics and toxicity have been extensively studied. The mushroom lectins are endowed with antiproliferative, antitumor, mitogenic, hypotensive, vasorelaxing, haemolytic, anti-HIV1 reverse transcriptase and immunopotentiating activities [3]. Some of the mushroom lectins, used in clinical diagnostics, are now commercially available. They include Agaricus bisporus lectin marketed by Sigma Aldrich Co. and EY Laboratories Inc., USA, Aleuria aurantia lectin marketed by Vector Laboratories Inc., USA, and Marasmius oreadus and Polyporus squamosus lectins marketed by EY Laboratories Inc., USA. In view of the many potential applications of mushroom lectins, investigation leading to purification and characterisation of them from diverse mushroom sources is a scientifically relevant exercise. In the present investigation, a novel lectin exhibiting no specificity for simple sugars, sugar derivatives, polysaccharides and glycoproteins tested was purified and partially characterised from the fruiting body of an edible mushroom Lentinus cladopus Lév.
2. Materials and Methods
2.1. Lectin Source
- Fresh fruiting bodies of the edible mushroom Lentinus cladopus Lév. (locally known as “tek-tek paan” in Manipuri), were collected during the period late August to October from Kakching in the Thoubal District, Manipur State, India as the source of lectin. The mushroom, shown in Figure 1, belongs to the family Polyporaceae of the order Agaricomycetes. The mushroom grows commonly on decaying stumps and roots of trees. The pileus of the mushroom is centrally depressed or funnel shaped. The scales are larger at the centre and smaller towards the margin. The colour is usually white to creamish white.
 | Figure 1. The fruiting bodies of mushroom Lentinus cladopus Lév. growing on the bark of a mango tree |
2.2. Chemicals
- All the saccharides and their derivatives and the glycoproteins used in the investigation were purchased from Sigma Chemical Company, St. Louis, MO, USA. DEAE-Cellulose, Q-Sepharose, Sephadex G-75, Sephadex G-100, bovine serum albumin (Mr 66kDa), chicken egg ovalbumin (Mr 44kDa), α-chymotrypsin (Mr 29kDa), lysozyme (Mr 18.4kDa) and blue dextran (Mr 2000kDa) were also obtained from Sigma Chemical Company, St. Louis, MO, USA. Medium molecular weight (MMW) protein markers were procured from Merck, India. All other chemicals and biochemicals used were of analytical grade. Purified de-ionized water was obtained through the purification system TKA Smart2Pure UV/UF (Niederelbert, Germany).
2.3. Preparation of Rabbit RBC
- Blood (1 mL) was withdrawn from the posterior marginal vein of rabbit (New Zealand White) external ear by a procedure performed in accordance with the Guide for the Care and Use of Laboratory Animals [4], causing minimal pain to the experimental animal. RBC was separated from the blood by a low-speed centrifugation, re-suspended in 50mM phosphate buffer pH 7.4 containing 0.9% NaCl (PBS) (5mL) with gentle mixing and then washed by centrifugation. This procedure of re-suspension and subsequent centrifugation was repeated four times to obtain washed RBC. The washed RBC (0.2mL) was diluted with PBS (10mL) to obtain 2% (v/v) RBC suspension.
2.4. Lectin Assay
- Lectin assay was carried out according to the method of Devi et al. [5] by two-fold serial dilution of 50μL of the lectin sample in a U-bottom 96-well microtitre plate with equal volume of PBS followed by addition of equal volume of the washed 2%(v/v) rabbit RBC suspension. After incubation for 1hr at 37°C, haemagglutination was scored visually. The lectin activity was taken as the reciprocal of the highest dilution of the lectin sample at which complete haemagglutination occurred. It was expressed in terms of haemagglutination unit (HAU) that is the minimum amount of lectin required for complete haemagglutination under the assay condition. Protein concentration was estimated by the method of Lowry et al. [6] using crystalline bovine serum albumin as the standard. The specific haemagglutination activity was expressed as HAU/mg protein.
2.5. Lectin Purification
- The lectin was purified from the fruiting body of the edible mushroom Lentinus cladopus Lév. by a procedure carried out at 0-8C unless otherwise stated. 10g of the coarse mushroom powder prepared from the air-dried fruiting bodies of the mushroom was soaked in 70mL of 20mM Tris-HCl buffer pH 7.8 containing 25mM NaCl, 0.1mM EDTA and 1mM β-mercaptoethanol. After soaking for 1hr, it was homogenised in a pestle and mortar under ice-cold condition. The homogenate was filtered through a double layered cotton cloth and then centrifuged at 17,000g for 30min at 4°C. The resulting light brown supernatant was treated with activated charcoal (90 mg/mL) for 15 min in ice-cold condition. After the charcoal treatment, the suspension was centrifuged and the resulting supernatant was filtered through glass wool to obtain a clear solution as the crude lectin preparation. The lectin preparation was then brought to initial 20% ammonium sulphate saturation at 0C. The resulting suspension was centrifuged at 17,000g for 30min at 4C and the supernatant obtained was then brought to 60 ammonium sulphate saturation at 0C. The suspension obtained was centrifuged, the pellet obtained as the ammonium sulphate (20-60%) fraction was re-dissolved in a minimum volume of 20mM Tris-HCl buffer pH 7.8 containing 25mM NaCl (TBS), and then dialysed extensively against the same buffer at 0C. The dialysed fraction was then subjected to discontinuous gradient Q-Sepharose chromatography using a column (1x4 cm, bed volume 4.2 mL), pre-equilibrated with TBS. The charged column was extensively washed and the bound protein was eluted by the same buffer at a flow rate of 30 mL/hr at increasing but different salt concentrations (0.1 to 0.5M). The peak fractions with haemagglutinating activity eluted at 0.2M NaCl were pooled, dialysed against de-ionized water and then lyophilised. The lyophilised powder was re-dissolved, dialysed extensively against 50mM phosphate buffer pH 6.8, and then subjected to anion exchange chromatography using a DEAE-cellulose column (1x4cm, bed volume 4.2mL) pre-equilibrated with the same buffer. The charged column was thoroughly washed, and then the bound proteins were eluted with the same buffer at a continuous gradient of 0.02 to 0.25M NaCl at a flow rate of 6mL/hr. Fractions having activity were pooled, dialysed extensively against TBS, and then subjected to a continuous gradient Q-Sepharose chromatography using a column (1x4cm, bed volume 4.2mL) pre-equilibrated with TBS. The charged column was extensively washed and then the bound protein was eluted with the same buffer at a continuous gradient ranging from 0.025 to 0.20M NaCl at a flow rate of 30mL/hr. Fractions with activity were pooled, extensively dialysed, and then lyophilised to obtain the purified lectin preparation. The activity and protein of the purified lectin preparation were determined to ascertain its specific activity.
2.6. SDS-PAGE
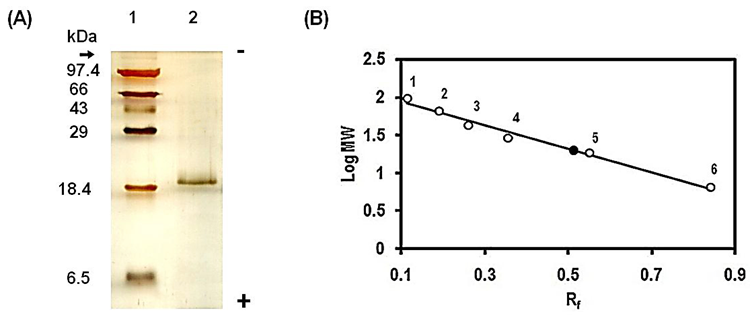
- The purified lectin preparation was subjected to 14% reducing SDS-PAGE by the method of Laemmli [7]. The electrophoresed protein bands were visualised by the silver staining method of Merril [8]. The MWM protein markers (Merck, India) were used as reference proteins. The relative subunit molecular weight of the purified lectin was determined using the standard plot of log Mr vs. Rf.
2.7. Native Molecular Weight Determination
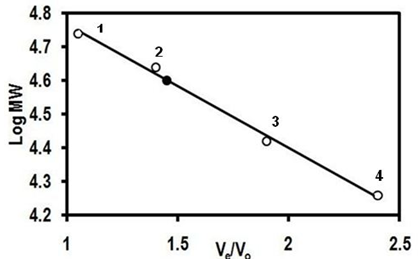
- The native molecular weight of the purified lectin was determined by molecular sieving gel chromatography under native condition. The lectin was eluted through a Sephadex G-75 column (82x0.9cm, bed volume 62 mL), pre-equilibrated with PBS. The void volume (Vo) of the gel column was determined using blue dextran (Mr 2,000kDa). The column was calibrated by using protein standards, viz. bovine serum albumin (Mr 66.43kDa), chicken egg ovalbumin (Mr 44.3kDa), α-chymotrypsin (Mr 29kDa) and lysozyme (Mr 18.4kDa). The elution of protein standards were monitored by the absorbance at 280nm and that of lectin by haemagglutination activity. The native molecular weight of the purified lectin was calculated from the standard plot of log Mr vs. Ve/Vo.
2.8. Haemagglutination Inhibition Assay
- Haemagglutination inhibition lectin assay was performed by a standard serial dilution procedure adopted by Devi et al. [5]. Each test carbohydrate solution (25μL) of fixed concentration [400mM in the case of sugar/sugar derivative, or 10% (w/v) in the case of polysaccharide/glycoprotein] was two-fold serially diluted with PBS (25μL) in a U-bottom microtitre plate. To each well, 25μL of the lectin sample under test having 4HAU of activity was added. After allowing 30min of incubation at 37C to facilitate carbohydrate-lectin binding, 25μL of 2% rabbit RBC suspension (v/v) was added to each of the wells. Corresponding control wells - one for carbohydrate control (lectin plus RBC only) and another for lectin control (carbohydrate plus RBC only) were always included. The haemgglutination pattern was visually examined after incubation for 1hr at 37C. The inhibitory concentration of the carbohydrate was recorded as the lowest concentration that can inhibit the complete haemgglutination.
2.9. Protein Identification by Mass Spectrometry
- The purified protein was first run on SDS-PAGE, stained with Coomassie blue and then the single protein band obtained was subjected to analysis for identification in the Proteomics International Facility, Bayliss Building, UWA Campus, Perth, Australia. The protein sample was trypsin digested and resulting peptides extracted according to standard technique of Bringans et al. [9]. The tryptic peptides were loaded onto an Agilent Zorbax 300SB-C18, 3.15 μm Agilent Technologies and separated with a linear gradient of water/acetonitrile/0.1% formic acid (v/v). The peptides were analyzed by electrospray ionization mass spectrometry using the Shimadzu Prominence nano HPLC system coupled to a 5600 triple TOF mass Spectrometer AB Sciex. Spectra were analysed to identify the protein using Mascot Sequence matching software Matrix Science applied to Ludwig NR database for Taxonomy: Fungi.
3. Results
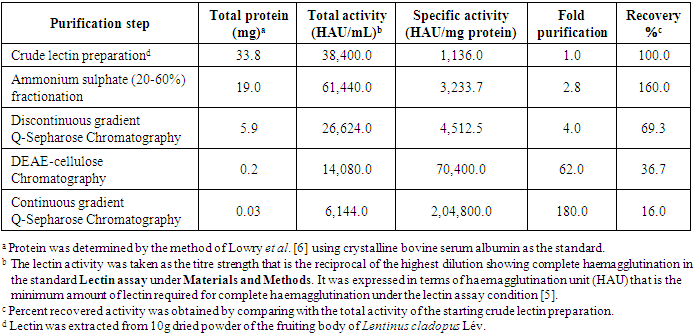
- A lectin was purified to electrophoretic homogeneity from the fruiting body of the edible mushroom Lentinus cladopus Lév. by successive steps of lectin extraction, ammonium sulphate (20-60%) fractionation, discontinuous gradient Q-Sepharose chromatography, DEAE-Cellulose chromatography and continuous gradient Q-Sepharose chromatography. The summary of the lectin purification is given in Table 1.
|
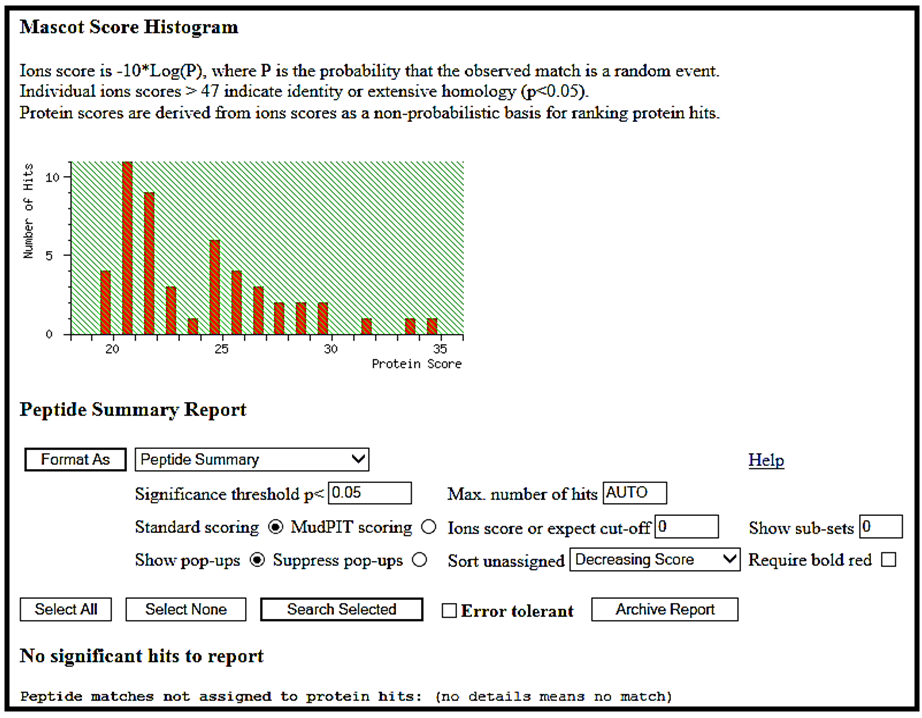 | Figure 4. Mascot score histogram of peptide fingerprinting analysis of the lectin purified from the fruiting body of Lentinus cladopus Lév. |
4. Discussion
- A lectin was purified from the fruiting body of edible mushroom, Lentinus cladopus Lév (Figure 1) by successive steps of (i) lectin extraction, (ii) ammonium sulphate (20-60%) fractionation, (iii) discontinuous gradient Q-Sepharose chromatography, (iv) DEAE-cellulose chromatography, and (v) continuous gradient Q-Sepharose chromatography. The final electrophoretically homogeneous lectin preparation obtained was purified by 180-folds with 16% recovery and had a specific lectin activity of 204,800 HAU/mg protein. No lectin has been purified and characterised till date from Lentinus cladopus Lév., though lectins from some other Lentinus species such as Lentinus squarrosulus [10] and Lentinus edodes (Berk.) Singer [11] were isolated and characterised earlier. The native molecular weight of the purified lectin, determined by gel filtration through Sephadex G-75, was found to be 40kDa (Figure 3). Though the molecular weights of lectins from mushrooms may be as low as 12kDa as reported for the lectin from the mushroom Flammulina velutipes [12] or may be as high as 190kDa as reported for the lectin from Laetiporus sulphureus [13], the native molecular weight of the lectin purified in the present investigation compares well with those of many mushroom lectins including those from Agrocybe aegerita (44kDa, [14]), Amanita pantherina (43kDa, [15]), Lentinus edodes (43kDa, [16]), and Psathyrella asperospora (41.8kDa, [17]). The subunit molecular weight of the purified lectin, determined by SDS-PAGE with reduction coupled with silver staining, was found to be 20kDa (Figure 2). Combining the result of native molecular weight determination by gel filtration with that of the subunit molecular weight determination by SDS-PAGE, it could be deduced that the lectin is a dimeric protein made up of apparently chemically identical subunits. This homodimeric subunit structural nature is in agreement with the earlier reports on other lectins from mushrooms including Agaricus edulis [18], Paxillus atrotomentosus [19], Pleurotus ostreatus [20], Boletus edulis [21], Lactarius flavidulus [22] and Clavaria purpurea [23]. The present Lentinus cladopus lectin is notable in that its haemagglutinating activity cannot be inhibited by the simple sugars and sugar derivatives tested. Some polysaccharides and glycoproteins tested also did not inhibit the haemagglutinating activity. As such, the lectin belongs to a group of a few mushroom lectins known to date showing haemagglutinating activity that is not inhibited by simple sugars. Some other lectins belonging to the group are Volvariella volvacea lectin [24], Mycoleptodonoides aitchisonii lectin [25], Lyophyllum shimeiji lectin [26], Agrocybe aegerita lectin [27], Armillaria luteo-virens lectin [28], Boletus venenatus isolectins [29] and Oudemansiella radicata lectin [30]. At this juncture, it is relevant to mention that the non-inhibition of the haemagglutinating activity of the Lentinus cladopus lectin by the externally added test carbohydrates might be due to absence of, or inaccessibility to, the required specific inhibitory covalent sugar sequence in them. The peptides obtained from tryptic digestion of the purified lectin were subjected to electrospray ionization mass spectrometry and the resulting spectra obtained were analyzed to identify the protein using Mascot sequence matching software Matrix Science applied to Ludwig NR database for Taxonomy: Fungi. The mass spectrometric analysis did not yield any significant hit (Figure 4). The result, therefore, showed that the lectin, purified from Lentinus cladopus Lév. in the present investigation, is a novel protein (lectin), not characterised earlier.
5. Conclusions
- A novel lectin was purified from an edible mushroom Lentinus cladopus Lév. by successive steps of lectin extraction, ammonium sulphate fractionation, discontinuous gradient Q-Sepharose chromatography, DEAE-cellulose chromatography and continuous gradient Q-Sepharose chromatography. The lectin was found to be a protein of molecular weight 40kDa made up of two apparently chemically identical subunits. Its lectin (haemagglutinating) activity was not inhibited by any of the simple sugars, sugar derivatives, polysaccharides or glycoproteins tested. Protein mass fingerprinting by electrospray ionisation mass spectrometry and subsequent Mascot database analysis showed the purified lectin to be a novel protein.
ACKNOWLEDGEMENTS
- Financial support in the form of University Research Fellowship awarded to Huidrom Rully by the Manipur University, Imphal-795003, Manipur, India is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML