-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2016; 6(3): 53-56
doi:10.5923/j.plant.20160603.01

Comparative Antibacterial Activity of Methanolic, Ethanolic and Aqueous Extract of Garcinia kola (Bitter kola) and Cola nitida (Kola nut)
Ezeigbo O. R. , Ejike E. N. , Nwachukwu I. , Ejike B. U.
Department of Biology/Microbiology, Abia State Polytechnic, Aba, Nigeria
Correspondence to: Ezeigbo O. R. , Department of Biology/Microbiology, Abia State Polytechnic, Aba, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Alternative herbal medicine has been used for centuries to treat various infections. Natural plants contain phytochemical properties similar to synthetic antibiotics. Due to multiple and repeated issues with antibiotic efficacy, it has become essential to evaluate biological properties of plants from different geographical origins. A comparative antibacterial activity of metanolic, ethanolic and aqueous extracts of Garcinia kola (bitter kola) and Cola nitida (Kola nut) were carried out against some test organisms (Salmonella species, Escherichia coli, Shigella species and Pseudomonas species). Agar well diffusion method was used in determining the antibacterial activity of the plant parts at different concentrations (100%, 75%, 50%, 25% and 12.5%) using methanolic, ethanolic and aqueous extracts of the samples. The results showed that the antibacterial activity of G. kola extract on the test organisms was significant for E. coli (p-values of 0.005); for Salmonella and Pseudomonas spp respectively (p-value of 0.001) and for Shigella spp (p-value of 0.016). For C. nitida, three bacterial isolates (Salmonella species, Shigella species and Pseudomonas species) were significantly inhibited having P-values of 0.000 for Salmonella and Pseudomonas respectively and p-value of 0.001 for Shigella species; however, E. coli was not significantly inhibited by C. nitida extracts (p-value of 0.203). Methanol extracts of the studied plants showed a wider spectrum of activity than ethanol and aqueous extracts. This study revealed the importance of these plant parts as a novel source of antimicrobial agents due to the increasing drug resistance among microorganisms. The infused extracts from the plants can therefore be considered by pharmaceutical industries in the production of cheap, affordable and available drugs for the cure of infections caused by these organisms in situations where expensive conventional drugs are unaffordable.
Keywords: Garcinia kola, Cola nitida, Methalolic, Ethanolic and aqueous extracts, Test organisms
Cite this paper: Ezeigbo O. R. , Ejike E. N. , Nwachukwu I. , Ejike B. U. , Comparative Antibacterial Activity of Methanolic, Ethanolic and Aqueous Extract of Garcinia kola (Bitter kola) and Cola nitida (Kola nut), International Journal of Plant Research, Vol. 6 No. 3, 2016, pp. 53-56. doi: 10.5923/j.plant.20160603.01.
Article Outline
1. Introduction
- The need for new antimicrobial agents is closely linked with the problem of emergence of strains that are resistant to most synthetic antibiotics. Despite the existence of potent antibiotic and antifungal agents, resistant or multi-resistant strains are continuously appearing, imposing the need for a permanent search and development of new drugs. It is therefore very necessary that the search for newer antibiotic sources be a continuous process. Natural plants contain phytochemical properties similar to synthetic antibiotics and therefore have been used in folk medicine to treat infections [1-3]. Plants are the cheapest and safer alternative sources of antimicrobials [4-6].G. kola commonly called bitter kola belongs to the family Guttiferae, grows in forest and cultivated for its economic importance. It is widely distributed in the tropics especially West African countries such as Nigeria and Sierra Leone. It is a tree plant of about 12m high with fruits produced from July to October. The fruits have subglobose, reddish yellow and about 2.5 inches in diameter, containing 2-4 ellipsoid brown seeds embedded in an orange coloured pulp [7]. The seeds have bitter taste, somewhat resembling that of the raw coffee bean, with residual slight sweetness. The seeds are chewed as stimulants among the natives. The seeds are used in folk medicine, many herbal formulations and have therapeutic benefits largely due to the presence of various secondary metabolites such as flavonoids, apigenin, kolaviron, biflavonoid-ametoflavone, saponins, tannins, resin [8, 9]. Scientific evaluations revealed that the stem bark and seeds are used for acute fever, cough, liver disorders and anti-vomiting agent [8, 10]. The seeds are also used as a remedy for inflammations of respiratory tract, bronchitis, throat troubles, stomach ache and gastritis [11, 12]. The seed extract is very efficacious for hepatitis, antiseptic and is active against Gram-positive and negative bacteria [8]. The decoction of the root is used as aphrodisiac, evacuant, anticancer and is also recommended for dysentery, headache, malignant tumours and respiratory ailments [10]. The root is chewed for cleaning teeth and toothache [8].C. nitida (Kola nut) belongs to Malvaceae, of the cocoa family. It is native to the main forest of tropical West Africa, but is now grown in many other humid climates like Sri Lanka. There are many species of kola plant but two species are of economic importance: Cola nitida and Cola acuminata. Kola nut exercises an exciting action in the brain without giving rise to any mental and physically painful effect. Moderate quantities are beneficial but harmful when eaten in excess by stimulating gastric acid secretion [13]. C. nitida contains both caffeine and tannin and therefore not advisable for individuals with stomach ulcers [14]. C. nitida is used for the treatment of morning sickness, migraine headache and indigestion and for cleaning teeth and gum [15]. It is used in controlling vomiting in pregnancy and as a stimulant to keep awake and withstand fatigue by students, drivers and other menial workers [16]. This study compares the antimicrobial activity of different extraction agents on G. kola (bitter kola) and C. nitida (kola nut) on some test organisms.
2. Materials and Methods
- Sample Collection and Processing: The seeds of G. kola and C. nitida were obtained from kola nut dealers at Umungasi market, Aba and authenticated by a plant taxonomist in the Department of Biology/Microbiology, Abia State Polytechnic, Aba. The seeds were washed with distilled water and dried at room temperature, dehusked and grinded into power with a mechanical grinder.Extraction of Plant Materials: The method of Aishmma and Mitscher [17] was used to obtain the plant extract. The active ingredients of each of the grounded particles of G. kola and C. nitida were extracted using methanol, ethanol and hot water respectively. Hundred grams (100g) of each of the grounded samples were added to 350ml distilled water, 95% of methanol and ethanol respectively and left to stand for 72 hours. The extracts were filtered separately using sterile Whatman’s no1filter paper and the filtrates were evaporated to dryness and stored in a deep freezer for further use.Test Organisms: The organisms used for this study include Escherichia coli, Salmonella spp, Shigella spp and Pseudomonas spp. The test organisms were obtained from the National Veterinary Research Institute, Umudike, Umuahia, Abia State. They were obtained as pure clinical isolates and were maintained on fresh nutrient agar slants and resuscitated for antibacterial testing. Antimicrobial Susceptibility Screening: The antimicrobial screening was carried out using agar well diffusion method as described by Cheesbrough [18]. One milliliter of sterile nutrient agar was poured into each of the petri-dishes. After setting, an overnight broth culture of Pseudomonas spp, Salmonella spp, Shigella spp and Escherichia coli were introduced into the surface of the sterile plates and sterile glass spreader was used for even distribution. Three wells of about 6mm were made asceptically with a sterile cork borer and 0.2ml of the extract of different concentrations was introduced into the wells. The extracts were allowed to diffuse into the medium for about I hour and then incubated at 37°C for 24 hours. The diameters of zones of inhibition were taken.Statistical Analysis: The data was analyzed using Statistical Package for Social Sciences (SPSS) version 20.0. The statistical tool employed was one way Analysis of Variance (ANOVA) to determine if there was any significance among the solutions. Statistical significance tests included the use of p-value to assess for the role of chance. In this study, p-value = 0.05 was used to disapprove the null hypothesis.
3. Results
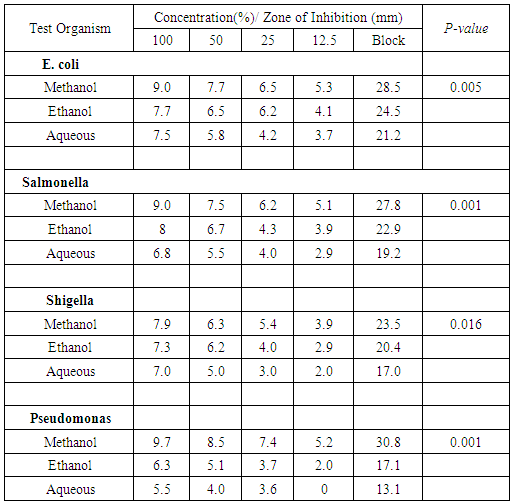
- Table 1 shows the antimicrobial activity of different concentrations of methanolic, ethanolic and aqueous extract of G. kola on some test organisms. From the result, all the test organisms were inhibited by the different extracts of G. kola. The methanol extract yielded the highest zones of inhibition ranging from 23.5-30.8mm for the test organisms. This is followed by ethanol extract ranging from 17.1-24.5mm while aqueous extract had the least inhibition from 13.1-21.2mm. The result also revealed that higher concentration produced higher zones of inhibition. The univariant test showed that all the test organisms were effectively inhibited by G. kola extracts having p-values of 0.005 for E. coli, 0.001 for Salmonella spp and Pseudomonas spp respectively and 0.016 for Shigella spp.
|
|
4. Discussion
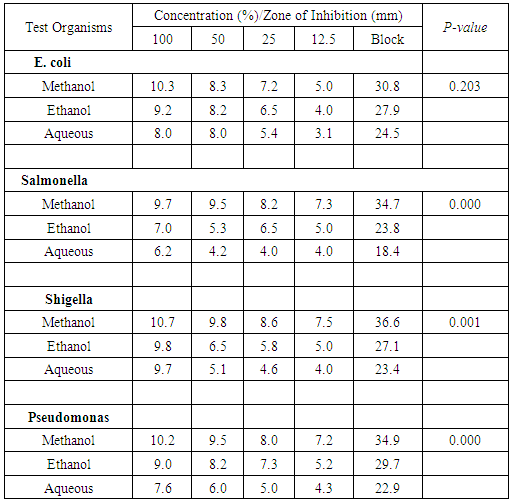
- Comparative analyses of the antibacterial activity of G. kola and C. nitida extracts using different extraction solvents were studied. The result revealed the antibacterial potency of the plants extracts studied. G. kola was able to inhibit the test organisms significantly while C. nitida, effectively inhibited Salmonella spp, Shigella spp and Pseudomonas spp but less significant on E. coli. These findings agree with the reports of some authors [19-22]. On the solvent used for extraction, the result revealed that methanol was a better extraction agent than ethanol and water. The methanol extract of the test plants were more effective in producing inhibition zone against the test organisms than ethanol and aqueous extracts. This finding also collaborated with the findings of some authors [21-24]. The bioactive agents were also better extracted at higher concentrations.
5. Conclusions
- Methanol, ethanol and aqueous extracts of G. kola and C. nitida possess antibacterial abilities. All the test organisms were significantly inhibited except E. coli that was less susceptible to C. nitida treatment. In terms of the susceptibility of the test organisms to the extraction solvents, methanol extract > ethanol extract > aqueous extract. Thus, methanol extraction of the test plants was more effective in producing inhibition zones on the test organisms than ethanol and aqueous extracts. The test plants therefore possess potentials for manufacturing potent drugs that could be used for the treatment of infections caused by test organisms such as typhoid fever, gastroenteritis, throat infections and cough.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the assistance of Dr. D. A. Awomukwu for the identification of the plant samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML