-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2016; 6(1): 13-19
doi:10.5923/j.plant.20160601.03

Isolation, Purification and Partial Characterization of Three Lectins from Tamarindus indica Seeds with a Novel Sugar Specificity
Makarim Elfadil M. Osman 1, Amna K. E. Awadallah 1, Emadeldin Hassan E. Konozy 2
1Department of Zoology, Faculty of Science, University of Khartoum, Khartoum, Sudan (Authors share equal contribution)
2Biotechnology Park, Africa City of Technology, Khartoum, Sudan
Correspondence to: Emadeldin Hassan E. Konozy , Biotechnology Park, Africa City of Technology, Khartoum, Sudan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lectins are carbohydrate binding proteins which attract great attention due to their wide functions and applications. At this investigation, we aimed to isolate, purify and partially characterize lectins from Tamarindus indica seeds. Upon fractionation of crude saline extract by varying concentrations of ammonium sulfate (30%, 60% and 80%) three lectins were isolated which were denoted EMtL3, EMtL6 and EMtL8, respectively. Both EMtL3 and EMtL6 were inhibited by glucose and GlcNAc (EMtL3 Minimum Inhibitory Concentration (MIC) = 0.045 and EMtL6 = 12.5 mM) and therefore, were purified on Sephadex G-75 as affinity matrix. EMtL8 has unique sugar specificity toward mannose and maltose (MIC=0.78 and 0.39 mM, respectively). Only EMtL3 and EMtL6 can agglutinate trypsin treated and untreated human RBCs with preference for AB type (512 unit) and A (20484 unit), respectively. The three lectins were unequally agglutinated rabbit erythrocytes. Native molecular weight of EMtL8 was 33 kDa and can form pentameric complex of 130 KDa at pH 5.0. EMtL3 subunit molecular weight is around 32kDa, while EMtL6 is 34 kDa. Both EMtL3 and EMtL6 were acid sensitive, they lost up to 40% of their activity at pH>6.0, they retained 50% activity after incubation with 5M urea for 2hr. EMtL6 is Zn+2 dependent. On testing these lectins for their capacity to agglutinate varying bacterial strains, all lectins could agglutinate Escherichia coli whereas only EMtL6 was able agglutinate Salmonella typhimurium and Staphylococcus aureus. To the best of our knowledge this is the first report on a legume lectin with rare sugar specificity toward mannose/maltose. The novel specificity of some lectin of the current investigation adds a mosaic piece to our current knowledge on plant lectin.
Keywords: Tamarindus indica, Isolectins, Mannose/maltose specific lectin, Bacteriostatic
Cite this paper: Makarim Elfadil M. Osman , Amna K. E. Awadallah , Emadeldin Hassan E. Konozy , Isolation, Purification and Partial Characterization of Three Lectins from Tamarindus indica Seeds with a Novel Sugar Specificity, International Journal of Plant Research, Vol. 6 No. 1, 2016, pp. 13-19. doi: 10.5923/j.plant.20160601.03.
Article Outline
1. Introduction
- Many plants express what so called lectins, the carbohydrate-binding proteins, the area of homologous proteins with ability to interact covalently and reversibly with glycoconjugates without altering their structure. This class of proteins gained much of interest since the 19th century [1], for their wide range of specificities and higher affinity toward oligosaccharides rather than simple sugar moieties [2]. It's well known that structurally different lectins can recognize and interact with the same haptens, and their specificity is directed toward non-plant origin glycans and glycol-conjugates [3, 4]. Glycans could attain much complicated architecture, and therefore suited and well adapted to carry biological codes, which can only be deciphered by lectins [5-7]. The recent evolutionary classification of lectin evolved to seven major families after they were only four [3, 8-10] and it's still in continuous growing. Tamarind is a legume tropical large plant of up to 24m tall. The word tamarind is derived from Arabic word "tamar" which means dry date fruits. Though this plant is thought to be indigenous to India, its habitation spans many parts of the globe [11]. The leaves, park and seeds of tamarind is of wide medical applications values. In particular, seeds extract is known to possess varying activities such as anti-venom, antioxidant, anticancer, antidiabetic, and antibacterial as well as eyes moisturizer [12].A lectin specific for N-acetylglucosamine was already isolated from seeds T. indica and structurally studied in relation to chitinase type III [13-16]. Our preliminary studies with T. indica seeds indicated the presence of a unique mannose/maltose agglutinating activity. Therefore, this investigation was undertaken to isolate this novel lectin as well as to further characterize the glucose/ N-acetylglucosamine lectins.
2. Materials and Methods
- Gel filtration molecular weight marker kit (MWGF70- IKT 098k6082) was bought from Sigma alderish, USA. Sephadex G-75 and 100 matrices were from Pharmacia, Sweden. All other materials were either of analytical grade or highest grade available. Tamarindus indica mature seeds were brought from local market.
2.1. Preparation of Tamarindus indica Seed Extract
- This was carried out essentially as described by Konozy et al [17]. In brief; 114g season fresh mature tamarind seeds were ground to fine powder using coffee grinder. Powder was mixed with butanol-1 (1g: 5ml) and stirred continuously for 4 h at 4°C. The defatted slurry obtained after removal of the organic layer by centrifugation was subjected to protein precipitation by the addition of equal volume of pre-chilled acetone with continuous stirring. The acetone dried powder thus obtained was subjected to extraction with physiological saline 0.145M (1g: 5ml) for 4 h at 4°C. Saline extracts which denoted hereafter as (FracA), was then fractionated by successive addition of varying percentages of solid (NH4)2SO4 30%, 60% and 80%, respectively. The precipitant obtained with every saturation was disolved in minimal amount of 0.145 mM NaCl then dialyzed exhaustively against ample amount of 0.145 mM NaCl till free of ammonium sulfate.
2.2. Protein Estimation
- Protein concentration was assayed by two different methods, UV absorption at 280nm and Lowry assay using BSA as the standard [18].
2.3. Carbohydrate Estimation
- Neutral carbohydrate content was estimated by phenol-sulfuric acid methods using D-glucose as the standard [19].
2.4. Hemagglutination Activity Assay (HA)
- Hemagglutination test was performed in ELISA micro-titer plates, in a final volume of 100 μL. Each well contained 50 μL of lectin solution and 50 μL of a 4% (v/v) of erythrocyte suspension of either trypsinized or untrypsinized human or animal RBCs. Agglutination was assessed after 1hr at room temperature. Hemagglutinating activity was expressed as titer, namely, the reciprocal of the highest dilution that gave a clear agglutination. The specific hemagglutinating activity was defined as titer per mg lectin.
2.5. Purifications and Molecular Weight Determination
2.5.1. Affinity Chromatography
- Sephadex G-75 packed in 2×8 cm column. Resin was equilibrated with physiological saline 0.145M and used as affinity matrix to purify lectin from fraction 30% and 60% [20]. Fractions were loaded onto the column separately. Column was saturated with protein after recycling for several times to ensure maximum retention. Column was washed with three bed volume with physiological saline till OD280 ≤0.02; the bound proteins were eluted with 100mM glucose. Fractions rich with lectin were pooled and concentrated using Millipore centrifugation devise (cut off 10kDa) and preserved at -20°C till further use.
2.5.2. Gel Filtration
- Sephadex G-100 packed into 1.5x70cm column was used to calculate the native molecular weight of eluted lectin from fraction 80%. The column was initially equilibrated with physiological saline then calibrated using 5 gel filtration standard protein markers [Blue Dextran 2000KDa, Albumin 66KDa, Carbonic Anhydrase 29KDa, Cytochrome C 12.4KDa and Aprotinin 6.5KDa] (MWGF70- IKT 098k6082). The gelfiltration was conducted initially at pH 7.0 then repeated at pH 5.0. Fractions of 3ml were collected at a flow rate of 1mL/min, fractions rich in lectin activity were pooled and concentrated using Millipore concentrating devise and preserved at -20°C till further use.
2.5.3. SDS-Polyacrylamide Gel Eectrophoresis (SDS-PAGE)
- Proteins were precipitated with TCA- acetone protocol. Sodium dodecyl sulphate polyacrylamide gel electrophoresis was performed according to Laemmli’s procedures [21]. The protein bands were visualized using Coomassie Brilliant Blue and silver nitrate staining [22].
2.6. Hemagglutination Inhibition Activity Assay
- The inhibition assays were performed in similar manner to the previously stated hemagglutination assays. 50mM of the desired carbohydrates [N-acetyl-glucosamine (GlcNAc), N-acetly-galactosamine (GalNAc), D-glucose, D-galactose, D-mannose, D-lactose, D-maltose, D-arabinose and D-dextrose] were used for testing their inhibitory effect on lectin. This test was done by mixing serialy diluted sugar with suitably diluted lectins followed by 2h incubation at room temperature, before addition of equal volume of 4% erythrocytes suspension.
2.7. Effect of pH on Lectin Activity
- 25µL of lectins aliquots were incubated with equal volume of 20mM buffers of varying pH ranging from 2-13; sample was incubated for 2h. pH was adjusted to 7.0 using 50mM of NaOH or HCl. Erythrocytes suspension were added and lectin activity was assayed.
2.8. Effect of Denaturing Agent on Lectin Stability
- Varying concentrations of urea 1, 2, 3, 4, 5 and 6M were used to study effect of denaturing agents on lectins activity. 25µL of urea was incubated with equal volume of lectin at room temperature for 2h, then the residual lectin activity was determined.
2.9. Effect of EDTA and Metal Ions on Lectin Activity
- Lectin solution was dialyzed overnight against 100mM of EDTA prepared in 0.145M NaCl. Lectin solution was then dialyzed against ample volume of physiological saline prepared in deionized water. Hemagglutination activity was assayed before and after EDTA incubation. Sample without EDTA treatment was considered as 100% activity. 25µL of 50mM divalent metal ions (Ca+2, Mg+2, Mn+2, Zn+2, Fe+2 and Hg+2) prepared in physiological saline were incubated with equal volume of EDTA treated lectin aliquots for 2h and lectins activity was determined.
2.10. Bacterial Growth Inhibition Assay
- The entire experiment was done under strict aseptic conditions. Five bacterial species of both gram positive and gram negative (Escherichia coli, Salmonella typhimurium, Shigella dysenteriae, Listeria monocytogenes and Staphylococcus aureus) were cultured in nutrient broth agar plates. Molten agar was poured into sterile Petri dishes and allowed to solidify in room temperature. Evaluation of effect of lectins on bacterial growth was performed in triplicates. Each set comprised of a negative control where respective bacterial strain is allowed to grow on agar in the absence of lectin, the test plate was composed of three 0.5cm circular filter papers that were aseptically saturated with each lectin, then placed in a triangular shape on top of each agar Petri dish. Approx. 20μL bacterial strain (1×108 cell/mL) was carefully dropped on the filter papers and incubated overnight. In the last agar Petri dish, lectin that was previously incubated with haptenic sugar was carefully added on the filter paper that was previously saturated with bacterial strain.
2.11. Bacterial Agglutination Assay
- Bacterial agglutination was performed in the same manner of hemagglutination. 1×108 cell/mL bacteria were 2 fold serially diluted to which 3 units of each lectin was added under aseptic conditions. Mixture was incubated for 30min and bacteria agglutination was monitored microscopically. The assay was repeated after incubating lectins with its haptenic carbohydrate.
3. Result and Discussion
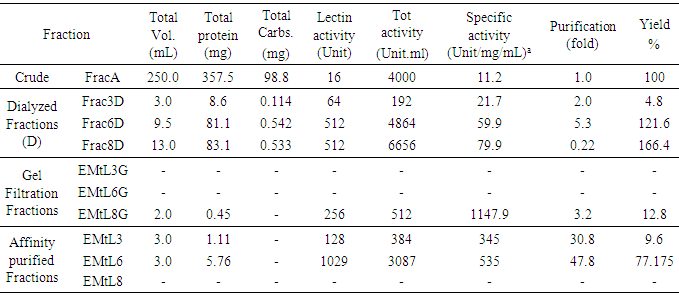
3.1. Purification of EMtLs
- Extraction of protein from Tamarindus indica seeds was very challenging due to the high lipid and pigments content especially that comes from the difficult to remove kernel peel. Therefore, defatting and depigmentation followed by dialysis were critical during lectin purification steps. To facilitate purifying the strong hemagglutinating activity (4000 U/mL, Table 2) from the crude seeds extract, successive fractionation by ammonium sulfate revealed dissemination of lectin activities at three varying precipitants obtained at different concentrations of the salt. This finding contradicted results reported by Grant and his colleagues in which only one lectin was present in seeds [23]. Each of the obtained ammonium sulfate precipitated protein fraction retained reasonably high hemagglutinating activity when tested against all human RBCs types. The purification processes of these lectins were summarized in Table 2. Interestingly, when the fruit pulp juice was fractionated in the same fashion with 30% ammonium sulfate saturation, a lectin activity specific for galactose [27], was detected (data not shown). The glucose specific lectins, EMtL3 and EMtL6, were purified in a single step using Sephadex G-75 as affinity chromatography [24-26] with concomitant 9.6% and 77.2% protein yield, respectively. Sequence and structural studies on varying glucose specific lectins from Tamarind suggested that they evolutionary belong to the chitin-binding lectin type III class [13-16]. As far as sugar specificity is a concern, and as Tamarind belongs taxonomically to the leguminosae, it is logical to correlate the lectins isolated from this plant to those ones isolated and studied from legume family [7, 28-31], however, to our surprise, EMtL8 revealed unusual and unique activity that was only inhibited by mannose/maltose. That way, EMtL8 resembles Calystegia sepium rhizomes lectin (Calsepa) and Dioscorea batatas lectin (Yam Tuber) [33-34]. EMtL8 was partially purified by gel filtration with 13% protein yield. Running gelfiltration experiment at two different pHs i.e. 7 and 5, exhibited varying oligomerization behaviour of lectin. At pH 7, lectin activity was detected at two peaks while a single peak activity was noticed at pH 5 (Figure 1).
|
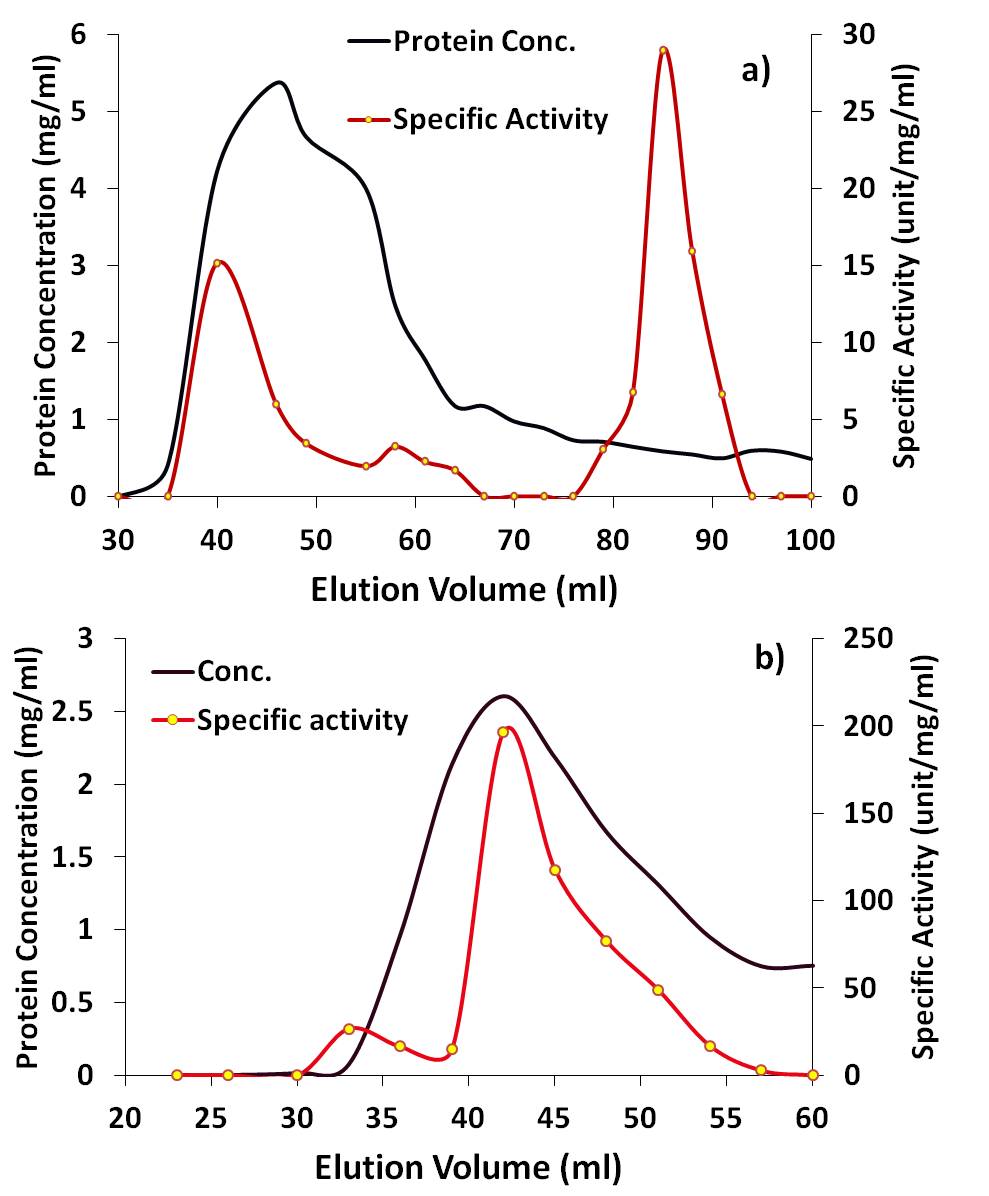 | Figure 1. Gel filtration of fraction 80%. a) 7.2mg were loaded onto 1.5×70cm Sephadex G-100 column, 3 mL fraction were collected at a flow ratr 1mL/min. b) 9mg of loaded proteins were eluted under acidic condition using 10mM acetate buffer (pH 5.0) |
3.2. Molecular Weight Determination
- Most of the studies on Chitin-lectin subunits molecular weight reported 34 kDa [13-16], which is in accordance with EMtL6 results, whereas, slightly lower band was noticed for EMtL3 (Figure 2). EMtL8 native molecular weight on gel filtration was determined at both pH 7 and 5. At the former case, two peaks correspond to 33 and 130 kDa were evident, whereas a single activity peak at around 130kDa was detected for the later. These results may suggest the presence of two subunits oligomerization for EMtL8. Such pH-dependent high order of oligomerization is well known as in case of Concanavalin A (ConA) a glucose specific lectin purified from the legume plant Canavalia ensiformis. Con A is a diamer under physiological pH and a complex of tetramer at acidic pH [35, 36].
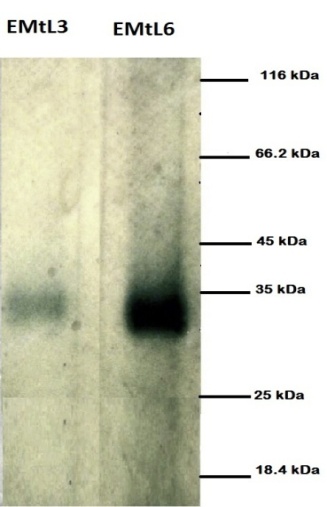 | Figure 2. 12.5% SDS-PAGE under reduced condition for EMtL3 and EMtL6. 10µL of each lectin were loaded. Molecular marker composed of 7 native proteins (β-galactosidase 116KDa, BSA 66.2KDa, Ovalbumin 45KDa, Lactate dehydrogenase 35KDa, REase E.coli 25KDa, β-Lactoglbuline 18.4KDa and Lysozme 14KDa) |
3.3. Hemagglutination and Sugar Specificity
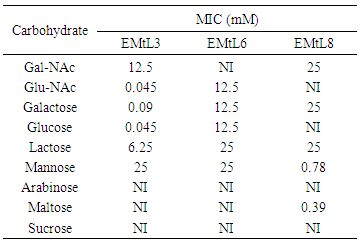
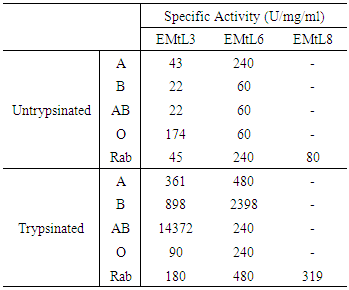
- The remarkable ability of plant lectins to agglutinate different types of human blood cells is well known [37]. EMtL3 and EMtL6 agglutinated all human blood types, however with clear preference toward certain blood type [38]. Like many other legume lectins, activities of both EMtL3 and 6 were enhanced several folds upon treatment of erythrocytes with trypsin [39] Table 3. On the other hand, EMtL8 could only agglutinate rabbit erythrocytes (Table 3). These results, again, are in good agreement with those results obtained for Calsepa lectin (C. sepium) [32]. EMtL6 and EMtL3 were inhibited by GlcNAc with MIC for equal to 0.045mM, whereas its counterpart EMtL6 was inhibited by the same sugar at a concentration 300 folds more than the one required for EMtL3 (MIC= 12.5). These results may clearly indicate that EMtL3 possesses extended binding site as compared to EMtL6. EMtL8 was mainly inhibited by mannose and maltose MIC= 0.78 mM and 0.39 mM respectively (Table 3). The distinct variations among these three lectins in terms of their behavior towards different inhibitory sugars may suggest distinct, yet obscure, physiological role(s).
|
3.4. Effect of pH, Urea, EDTA and Divalent Metals on EMtL3 and 6 Lectins Activity and Stability
- Examining the activity and stability of EMtL3 and 6 lectins toward different pH buffers showed that they are acid sensitive lectins. They lost ~ 40% of their activity at pH below 6.0 (Figure 4). EMtL3 and EMtL6 are considered stable lectins as they only lost 50% of their residual activity after incubated at 5M of urea for 2hrs (Figure 3). Stability of EMtL3 and EMtL6 to urea matches that of Erythrina speciosa lectin (EspecL) [17]. However, the former is a metallo-protein whose function can be altered by EDTA and Zn+2. EMtL6 activity was totally abolished upon incubation with EDTA; the activity was regained after incubating protein with 50mM of metal ions. 5 folds increase in lectin activity was noticed upon addition of Zn+2. EDTA had no effect on EMtL3 activity; which may indicate that it’s either not a metallo-protein or the metal is firmly bound to the interior side of lectin that it was difficult to be removed by chelating agents. Activity of lectins was increased 3 folds by Ca+2, Mg+2, Mn+2 and Zn+2 (Figure 5).
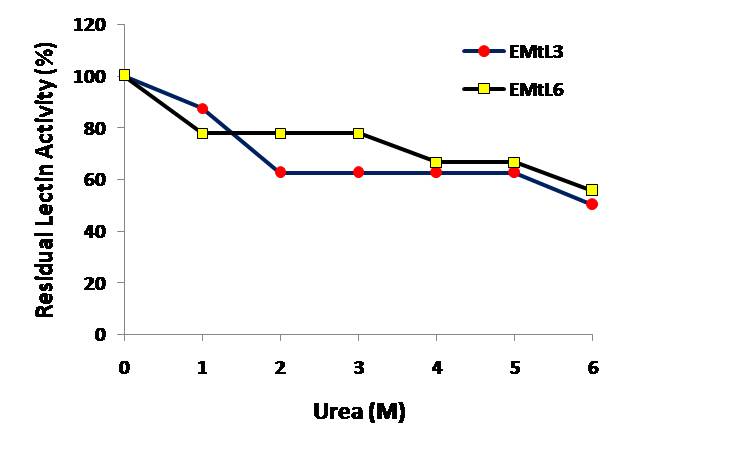 | Figure 3. Residual Effect of denaturing agent (urea) on lectins activity and stability |
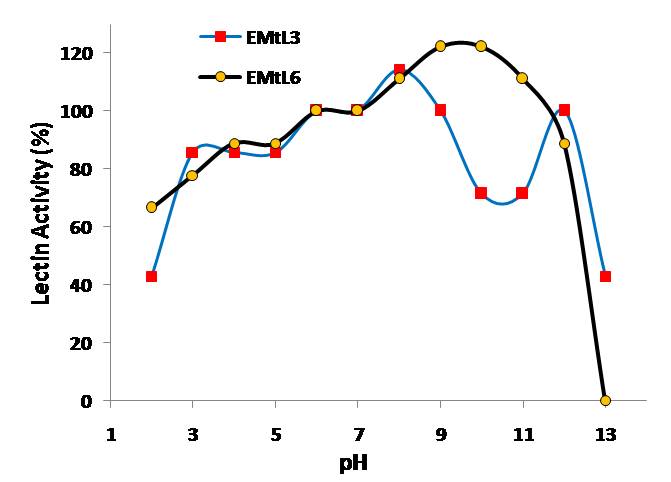 | Figure 4. Effect pH on lectins activity |
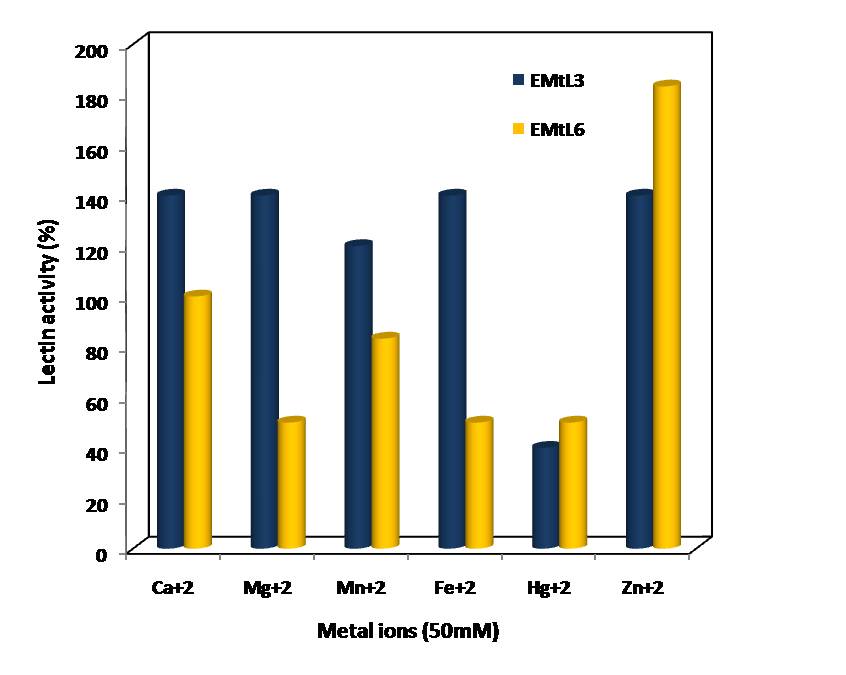 | Figure 5. Effect of metal ions on EMtL3 and EMtL6 lectins activity |
3.5. Bacterial Inhibition and Agglutination Assay
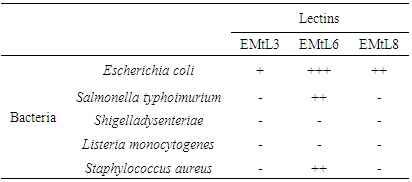
- The only available data on tamarind seed biological effect on bacterial strains was obtained from organic methanolic extracts and other organic compounds [40-42]. The three isolectins didn't inhibit agar-plated bacterial growth of E. coli, S. typhimurium, S dysenteriae, L. monocytogenes and S. aureus at any concentration. However, on performing the agglutinating test followed by microscopic studies, the three lectins were found to agglutinate all human non-pathogenic E. coli [27]. On the other hand, EMtL6 showed more capability to agglutinate S. typhimurium and S. aureus (Table 4). Thus these lectins could be considered as to be having bacterostatic effect rather than bactericidal effect on tested bacterial strains.
|
4. Conclusions
- Several research studies reported that T. indica is rich with Chitinase lectin type III, nevertheless there isn't a single article indicated the presence of mannose/maltose binding lectin. Systematic isolation and purification led to its discovery and allowed more detailed comparison between the isolectins in terms of biological and physical characterizations. Our future plan is to identify the evolutionary relationship between these lectins and other members of the same sugar specificity.
ACKNOWLEDGEMENTS
- We wish to acknowledge Prof. Atif A Elagib, Deputy Director, National Institute for Research, Khartoum, for his support and laboratory help during this project.
References
| [1] | Sharon, N., 2008, Lectins: past, present and future, Biochem.Soc.Trans., 36, 1457-1460. |
| [2] | Dodd, R.B., and Drickamer, K., 2001, Lectin- like protein in model organisms: Implications for evolution of carbohydrate- binding activity, Glycobiol, 11(5), 71R-79R. |
| [3] | De Hoff, P.L., Brill, L., Hirsch, A.M., 2009, Plant Lectin: the ties that bind in the root symbiosis and plant defense, Mol. Genet. Genomics, 282, 1-15. |
| [4] | Sharon, N., and Lis, H., 2004, History of lectins: from hemagglutinin to biological recognition molecule, Glycobiol., 14(11), 53R-62R. |
| [5] | Murphy, P.V., Andre, S., Gabius, H.J., 2013, The third dimension of reading the sugar code by lectins: Design of glycoclusters with cyclic scaffolds as tool with the aim to define correlation between spatial presentation and activity, Mol., 18, 4026-4053. |
| [6] | Ambrosi, M., Cameron, N.R., Davis, B.G., 2005, Lectins tools for molecular understanding of the glycocode, Org Biomol. Chem., 3, 1593-1608. |
| [7] | Gabius, H.J., Andre, S., Barbero, J.J., Romero, A., Solis, D., 2011, From lectin structure to functional glycomics: Principle of sugar code, Trends Bioch. Sci., 36(6), 298-313. |
| [8] | Peumans, W.J., Van Damme, E.J.M., 1998, Plant Lectins: Versatile proteins with important perspectives in biotechnology, Biotech. Gene Eng. Rev., 15, 199-228. |
| [9] | Parijs, J.V., Broekaert, W.F., Goldstein, I.J., Peumans, W.J., 1991, Hevein an antifungal protein from rubber-tree (Hevea brasiliensis), Planta., 83(2), 258-264. |
| [10] | Wang, H., and Ng, T.B., 1998, Ribosome inactivating protein and lectin from bitter melon (Momordica charantia) seeds: Sequence comparison with related proteins, Biochem Biophys. Res. Commun., 253, 143-146. |
| [11] | Bagul, M., Sonawane, S.K., Arya, S., 2015, Tamarind seeds: Chemistry, technology, applications and health benefits: a review, Ind. F. Indus., 34(3), 28-35. |
| [12] | Kuru, P., 2014, Tamarindus indica and its health related effects, Asian Pasif. J. T. Biomed., 4(9), 616-681. |
| [13] | Patil, D.N., Datta, M., Chaudhary, A., Tomar, S., Kumar, S.A., 2009, Isolation, purification, crystallographic studies of chitinase from tamarind (Tamarindus indica) seeds, Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun., 65, 343-345. |
| [14] | Rao, D.H., and Gowda, L.R., 2008, Abundant class III acidic chitinase homologue in tamarind (Tamarindus indica) seeds serves as the major storage protein, J. Agric. Food. Chem., 56, 2175-2182. |
| [15] | Datta, M., and Tomar, S., Molecular cloning and structural characterization of catalytic domain of class III chitinase from Tamarindus indica, I. J. L. S. T., 3, 16-28. |
| [16] | Patil, D.N., Datta, M., Dev, A., Dhindwal, S., Singh, N., 2013, Structural investigation of a novel N-Acetyl Glucosamine binding Chi-lectin which reveals evolutionary relationship with class III chitinases, PLOS ONE. 8(5), e63779. |
| [17] | Konozy, E.H.E., Bernardes, E.S., Rosa, C., Faca, V., Greene, L.J., Ward, R.J., 2003, Isolation, purification and physiochemical characterization of a D- galactose-binding lectin from seeds of Erythrina speciosa, Acrh. Biochem. Biophys. 410, 222-229. |
| [18] | Lowry, O.H., Rosebrough, N.J., Fan, A.L., Randal, R.J., 1951, Protein measurement with the Folin-phenol reagent, J. Bio. Chem., 193, 265-275. |
| [19] | Dubois, M., Gilles, K., Hamilton, J.K., Rebers, P.A., Smith, F., 1956, A Colorimetric method for the determination of sugars and related substances, Anal. Chem., 28, 350-356. |
| [20] | Biswas, S., Agrawal, P., Saroh, A., Das, H.R., 2009, Purification and mass spectrometric characterization of Sesbania aculeate (Dhaincha) stem lectin, Prot. J., 28(9-10), 391-399. |
| [21] | Leammli, U.K., 1970, Cleavage of structural proteins during assembly of the head of bacteriophage T4, Nat., 277, 680-685. |
| [22] | Blum, H., Beier, H., Gross, H.J., 1987, Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels, Electroph., 8(2), 93-99. |
| [23] | Grant, G., More, L.J., Mckenzie, N.H., Donword, P.M., Stewart, J.L., Telek, L, Pusztai, A., 1991, A Survey of nutritional and hemagglutination properties of several tropical seeds, Livestock Res. Rural Develp., 3(3), 24-34. |
| [24] | Sasmal, D., Guhathakurta, B., Ghosh, A.N., Pal, C.R., Datta, A., 1999, Purification of a mannose/glucose-specific hemagglutinin/lectin from a Vibrio cholerae O1 strain, Immunol. Med. Microbiol., 23(3), 221-7. |
| [25] | Correia, M.T., and Coelho, L.C., 1995, Purification of a glucose/mannose specific lectin, isoform 1, from seeds of Cratylia mollis Mart. (Camaratu bean), Appl. Biochem. Biotechnol., 55(3), 261-73. |
| [26] | Kalsi, G., Das, H.R., Babu, C.R., Das, R.H., 1992, Isolation and characterization of a lectin from peanut roots, Biochim. Biophys. Acta. 1117(2), 114-9. |
| [27] | Rodriquez, R.C., Henandez-Cruz, P., Giles-Rios, H., 2001, Lectins in fruits having gastrointestinal activity: Their participation in the hemagglutinating property of Echerichia coli 0157:H7, Arch. Med. Res., 32, 251-257. |
| [28] | Loris, R., Hamelryck, T., Bouckaert, J., Wyns, L., 1998, Legume lectin structure, Biochimica. Biophysica. Acta. 383, 9-36. |
| [29] | Lioi, L., Galasso, I., Santantonio, M., Lanave, C., Bollini, R., Sprvoli, F., 2006, Lectin gene sequences and species relationships among cultivated legumes, Genet. Reso Crop. Evol., 53, 1615-1623. |
| [30] | Manoj, N,, and Suguna, K., 2001, Signature of quaternary structure in the sequence of legume lectins, Prot. Eng., 14(10), 735- 745. |
| [31] | Grandhi, N.J., Mamidi, A.S., Surolia, A., 2015, Pattern recognition in legume lectins to extrapolate amino acid variability to sugar specificity, Adv. Exp. Med. Biol., 842, 199-215. |
| [32] | Peumans, W.J., Winter, H.C., Bemer, V., Van Leuven, F., Goldstein, I.J., Turuffa-Bachi, P., Van Damme, E.J.M., 1997, Isolation of a novel plant lectin with an unusual specificity from Calystegia sepium, Glycocon. J., 14, 259-265. |
| [33] | Van Damme, E.J.M., Barre, A., Verhaert, P., Rouge, P., Peumans, W.J., 1996, Molecular cloning of the mitogenic mannose/maltose-specific rihizome lectin from Calystegia sepium, FEBS Letters, 397, 352-356. |
| [34] | Gaidamashvili, M., Ohizumi, Y., Lijma, S., Takayama, T., Ogawa, T., Muramoto, K., 2004, Characterization of Yam Tuber storage protein from Dioscorea batatas exhibiting unique lectin activity, Amir. Soci. Bioch. Mol. Bio., 279(25), 26028-26035. |
| [35] | Zand, R., Agrawal, B.B., Goldstein, I.J., 1971, pH dependant conformational changes of concanavalin A. Proc. Nalt. Acad. Sci. USA., 68(9), 2173-2176. |
| [36] | Auer, H.E., and Schilz, T., 1984, pH dependant changes in properties of concanavalin A in the acid pH range, Int. J. Pept. Prot. Res., 24(5), 462-471. |
| [37] | Hamid, R., and Masood, A., 2009, Dietary lectins as disease causing toxicants, Pakistan. J. Nut., 8(3), 293-303. |
| [38] | Vasconcelos, I.M., and Oliveira, J.T.A., 2004, Antinutritional properties of plant lectins, Toxicon, 44, 385-403. |
| [39] | Konozy, E.H., 2012, Characterization of D-galactose binding lectin from seeds of Erythrina lysistemon, Glo. Adv. Res. Biochem. Bioinform., 1(1), 007-018. |
| [40] | Muthu, S.E., Nandakumar, S., Rao, U.A., 2005, The effect of methanolic extracts of Tamarindus indica Linn.on the growth of clinical isolates of Burkhoderia pesudomallei, Indian J. Med. Res., 122, 525-528. |
| [41] | Ghelardi, E., Tavanti, A., Celandroni, F., Lupetti, A., Blandizzi, C., Boldrini, E., Campa, M., Senesi, S., 2000, Effect of a novel mucoadhesive polysaccharide obtained from tamarind seeds on the intraocular penetration of gentamicin and of loxacin in rabbits, J. Antimicrobial Chemotherapy, 46, 831-834. |
| [42] | Ghelardi, E., Tavanti, A., Davini, P., Celandroni, F., Salvetti, S., Parisio, E., Boldrini, E., Senesi, S., Campa, M., 2004, A mucoadhesive plymer extracted from Tamarind seed improves the intraocular penetration and efficacy of Rufloxacin in tropical treatment of experimental bacterial keratitis, Antimicro .Ag. Chemoth., pp. 3396-3401. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML