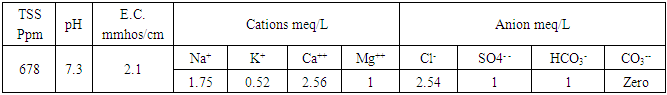-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2015; 5(5): 136-156
doi:10.5923/j.plant.20150505.06

Application of Salicylic Acid and Zinc Improves Wheat Yield through Physiological Processes under Different Levels of Irrigation Intervals
Mahmoud R. Sofy
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt
Correspondence to: Mahmoud R. Sofy, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Water stress, one of the environmental stresses, is the most significant factor restricting plant production on majority of agricultural fields of the world. Wheat is grown on arid-agricultural fields and drought often causes serious problems in wheat production in these fields. So, the present study was conducted to investigate whether the application of salicylic acid (SA) and zinc (Zn) could regulate the relative water content (RWC), membrane stability index (MSI%), electrolyte leakage (EL), soluble carbohydrate content, soluble protein content, proline content, activities of antioxidant non enzymes and enzymes as well as ameliorate the adverse effects of drought stress on wheat. The magnitude of reduction, increased by increasing the drought level. Drought stress at grain filling stage significantly decreased plant height, spike length, number of grains/spike, 1000-grain weight, relative water content, but significant increases were observed in the activities of superoxide dismutase (SOD), peroxidase (POX), catalase (CAT) and glutathione reductase (GR) in wheat plants. Both salicylic acid or zinc treatments had greater changes in most of the assayed parameters. The adverse effects of drought as regards the growth characters and yield components were significantly mitigated by SA and Zn supplement. Application of SA or caused great variations in the activities of antioxidant enzymes. Under the normal irrigation condition, addition of SA or Zn markedly increased the activity of both SOD and CAT, however the activity of both POX and GR was significantly decreased. Addition of SA or Zn markedly reduced the increases in the activities of SOD, POX, CAT and GR observed in drought stressed plants. Statistically, Pearson correlation showed there were positive correlations between weight of 1000 grains and morphology, biochemical assay. However, there was no correlation between weight of 1000 grains with proline content and GR activity. Generally, it could be concluded that SA or Zn has (to more extent) a beneficial regulatory role in plants grown under drought stress conditions.
Keywords: Irrigation interval, RWC, MSI, Salicylic acid, Zinc, Antioxidant enzyme activity
Cite this paper: Mahmoud R. Sofy, Application of Salicylic Acid and Zinc Improves Wheat Yield through Physiological Processes under Different Levels of Irrigation Intervals, International Journal of Plant Research, Vol. 5 No. 5, 2015, pp. 136-156. doi: 10.5923/j.plant.20150505.06.
Article Outline
1. Introduction
- Agronomic management factors, for example, irrigation plays important roles in experiencing this the maximum potential of productivity in crop plants. Drought stress can impact the progression of wheat plants and also the accumulation of active substances within their organs [1].Moderate drought stress may enhance the power of secondary metabolites in plants, while moderate drought stress is known to improve the concentration of secondary metabolites in plants [2, 3]. The main reason from the environmental stress, for example, drought inhibits the growth and photosynthetic abilities of plant may be the disturbance within the balance between the production of reactive oxygen species (ROS) along with the antioxidant defence, causing accumulation of ROS, which induces, damages protein, membrane lipids, oxidative stress and other cellular components [4].Wheat is an important crop in Egypt, where its production isn't enough to satisfy the demand for it. The crop is responsive to the timing of a water deficit period rather than the total reduction of applied irrigation water. Exposing wheat plants to high moisture stress, depressed seasonal consumptive use and grain yield [5]. The quantity of wheat yield reduction because of water stress is affected by the stage of grain development, where the early grain development stage is more susceptible to water stress than latter grain development stage. El-Kholy et al. [6] stated that, irrigation practices and irrigation water are factors which have always limited wheat productivity. The recommended number of irrigations at the reproductive stages and also the vegetative have to be applied suitably and timely for much better yields. Salicylic acid (SA) is an endogenous growth regulator of phenolic nature, which participates in the regulation of physiological processes in plant [7]. Also, participation in signal regulation of gene expression in the course of leaf senescence [8]. Generally, salicylic acid and its derivatives like acetylsalicylic acid have a significant impact on the various aspects of the plant life [9].Application grains with acetylsalicylic acid improved the drought tolerance in wheat [10]. Many studies report the protective effects of SA on plants against drought [11] and osmotic stress [12]. Salicylic acid regulates physiological and biochemical properties of plants under abiotic stress and it is also important in disease resistance [13].Zinc (Zn) is an important plant micronutrient that is involved with many physiological functions, carbohydrate and protein synthesis [14]. The decrease of zinc on plant has been associated with the drought stress caused by decreases in soil, water and therefore, limitation of root growth [15]. The use of zinc under drought conditions would influence crop quality and yield. It plays an important role in ionic balance and regulating stomata in crops to reducing the harmful results of drought [16] and also has protective effects on photo oxidative damage caused by ROS [17].The influence of drought were more serious during reproductive stage than a vegetative stage in wheat [18]. So, drought conditions coincide with the limit grain yield and grain filling period. The grain of wheat with SA either application SA and Zn can affect the sensitivity of wheat. The aim of the present work was to investigate the effectiveness of salicylic acid (SA) and zinc (Zn) in alleviating the negative effects of drought stress. Because of this we hypothesized that SA and Zn can mitigate the adverse effects of drought on yield components and physiological characteristics.
2. Materials and Methods
- These experiments were carried out in Botanical farm, Fac. of Sci., Al-Azhar Univ. Cairo, Egypt. The grains of wheat plant (Triticum aestivum var. Giza 168) were obtained from the Agricultural Research Centre, Ministry of Agriculture, Giza, Egypt. Soil samples were taken at the depth of 30 cm before planting for physical and chemical analysis as shown in (Tables 1 & 2) according the methods of Nelson and Sommers [19].
|
|
2.1. Determination of Relative Water Content (RWC)
- Leaf discs of 6 mm diameter were weighed to determine the fresh weight (FW), soaked in distilled water at 25°C for 4 h to determine the turgid weight (TW), then oven dried at 80°C for 24 h to determine the dry weight (DW). The relative water content was determined by following the method of Turner and Kramer [20], using the following equation:
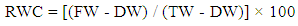
2.2. Membrane Stability Index (MSI%)
- MSI was estimated using conductivity meter. MSI calculated using the formula described by Sairam [21]. MSI was calculated using the following formula:
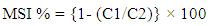
2.3. Electrolyte Leakage (EL)
- The total leakage of inorganic ions from the leaves was determined using the method of Sullivan and Ross [22]. Twenty leaf discs were placed in a boiling tube containing 10 ml deionized water and the electrical conductivity (EC1) was recorded. The contents were then heated to 45-55°C for 30 min each in a water bath and the electrical conductivity (EC2) was recorded. The sample was boiled at 100°C for 10 min and the electrical conductivity (EC3) was recorded. Electrolyte leakage was calculated using the formula:
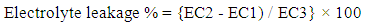
2.4. Phytochemical Contents
2.4.1. Determination of Soluble Carbohydrate Content
- Soluble carbohydrate content was determined in aqueous solution with anthrone sulfuric acid reagent according to Umbriet et al. [23] using glucose as a standard. To extract water-soluble carbohydrates, a known weight (0.1 g dry weight) of grains powder was boiled in distilled water in a water bath for 1 h. The extracts were then cooled and filtrated through a centered glass funnel. A total of 0.5 ml of each extract was mixed with 4.5 ml of anthrone reagent (0.2 g anthrone, 8 ml absolute ethyl alcohol, 30 ml distilled water and 100 ml sulfuric acid). The mixture was then boiled in a water bath for 7 min. After cooling, the developed blue green color was measured at 620 nm against the blank.
2.4.2. Determination of Soluble Protein Content
- The soluble protein content of grains was determined according to Lowery et al. [24] using bovine serum albumin as a standard. Samples (0.1 g dry weight) were extracted in10 ml distilled water for 2 h at 60°C. The extracts were centrifuged and the supernatants were collected. One ml of each extract was added to 5 ml of alkaline reagent (50 ml 2% Na2CO3 prepared in 0.1 N NaOH and 1 ml 0.5% CuSO4.5H2O prepared in 1% sodium potassium tartarate) and mixed thoroughly, then allowed to stand for 10 min. A total of 0.5 ml of folin phenol reagent diluted 1:2 (v/v) was then added and mixed immediately. After 30 min, the extinction against appropriate blank was measured at 700 nm.
2.5. Determination of Stress Response Factors
2.5.1. Determination of Ascorbic Acid (ASC)
- The total ascorbic acid content was estimated using folin phenol reagent according to Jagota and Dani [25].
2.5.2. Determination of Glutathione (GSH)
- Glutathione was extracted by grinding 0.5 g of plant tissues in 1% picric acid (w/v) under cold condition. After centrifugation at 10,000g for 10 min, the supernatant was collected immediately for assay. Glutathione was estimated according to Beutler et al. [26].
2.5.3. Determination of Lipid Peroxidation
- Lipid peroxidation was determined by estimating the malondialdehyde (MDA) content according to Herna´ndez and Almansa [27]. Fresh weight samples (500 mg) were homogenized in 5 ml of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000g for 20 min at 4°C. One ml aliquot of the supernatant was mixed with 3 ml of 0.5% thiobarbituric acid (TBA) prepared in 20% TCA and incubated at 90°C for 20 min. After stopping the reaction in an ice bath, samples were centrifuged at 10,000 g for 5 min. The supernatant absorbance at 532 nm was then measured. After subtracting the non-specific absorbance at 600 nm.
2.5.4. Determination of Proline Content
- Grains were hand-homogenized in 3% of sulfosalicylic acid and centrifuged at 3000g at 4°C for 10 min. The supernatants were used for proline estimation according to Bates et al. [28].
2.5.5. Determination of Total Phenolic Compounds
- Total phenolics were measured with the Folin–Ciocalteu reagent according to Dai and Mumper [29]. Twenty five ml of the extract was mixed with 110 ml Folin–Ciocalteu reagent, 200 ml 20% sodium carbonate and 1.9 ml distilled water, and placed at 60°C for 30 min. Optical density was measured with a spectrophotometer at 750 nm. A standard curve was constructed with different concentrations of gallic acid.
2.6. Determination of Antioxidant Enzymes
- Protein enzymes were extracted according to the method of Mu Kherjee and Choudhuri [30]. Superoxide dismutase (SOD), Peroxidase (POX), Catalase (CAT) activity and Glutathione reductase (GR) activities were assayed using the methods of Dhindsa et al. [31], Bergmeyer [32], Chen et al. [33] and Karni et al. [34], respectively.
2.7. Statistical Analysis
- The sample size was calculated according to Raosoft and all statistical calculations were done using SPSS (statistical package for the social science version 20.00) statistical program at 0.05 level of probability [35]. Quantitative data with parametric distribution were done using analysis of variance the one-way ANOVA and Post hoc-LSD tests (the least significant difference). The confidence interval was set to 95% and the margin of error accepted was set to 5%. The p-value was considered non-significant (NS) at the level of >0.05, significant at the level of <0.05, 0.01 and highly significant at the level of <0.001. The Pearson linear correlation coefficient, automatic and linear modelling and discriminant analysis were estimated to show the relationship between quantitative parameters [36].
3. Results and Discussion
3.1. Growth Parameters
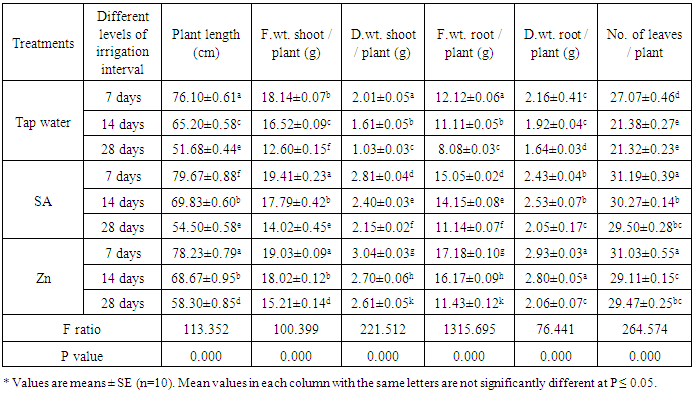
- The results of the present work (Table 3) revealed that, different levels of the irrigation interval of wheat plants every 14 and 28 days resulted in significant decreases in shoot length, fresh and dry weight of both shoots and roots and the number of leaves/plant when being compared with plants grown in normal non-stress condition. The magnitude of reduction, increased by increasing irrigated water days. These results are in agreement with those obtained by Raza et al. [37] and Yan and Shi [38], they reported that drought stress decrease wheat plant height. Also, Praba et al. [39] demonstrated that, in wheat there was a significant decrease in leaf fresh weight, leaf area, dry weight, plant height and shoot dry weight after vegetative stage drought. The obtained results (Table 3) revealed also that, treated wheat plants with SA or Zn resulted in significant increases in shoot length, fresh and dry weight of both shoots and roots and the number of leaves/plant as being compared with plants grown in non- or stress condition. Since, Anosheh et al. [40] reported that after the exogenous applications of salicylic acid reduced the harmful effects of drought. The positive effects of foliar spray of SA and Zn on vegetative growth of wheat under Irrigation interval were in harmony with the results observed by some investigators [16, 41, 42].
3.2. Yield and Yield Components
- Results in Figs. (1-5) revealed that, different levels of the irrigation interval of wheat plants every 14 and 28 days resulted in significant decreases in the number of tillers and spikes/plant as well as the number of grains/plant and weight of 1000-grain when being compared with plants grown in normal non-stress condition. At high number of grains/spike leads to higher grains yield in plants [43], but drought stress significantly reduced grain yield [44]. In the experiment of Kanani et al. [18] on wheat, grain yield and many of yield components significantly affected by drought stress on reproductive growth stage. A drought stress induced decrease in 1000-grains weight was previously reported by Farooq et al. [10] and Beigzadeh et al. [45].Also, Figs. (1-5) revealed that, the number of tillers and spikes/plant as well as the number of grains/plant and weight of 1000-grains were highly significantly increased in response to the treatments with SA or Zn with normal condition. Except interactive effects irrigation interval every 7 days with Zn revealed an insignificant increase in the number of spikes/plant. The obtained results in Figs. (1-5) revealed also that, treated wheat plants with SA or Zn resulted in significant increases in the number of tillers and spikes/plant as well as of grains/plant and weight of 1000-grains when irrigated water every 14 and 28 days. Except at irrigation interval every 28 days + either SA or Zn resulted in, insignificant increases in the number of tillers/plant. These outcomes are in agreement with Farooq et al. [10] and Monjezi et al. [16]. According to results, Sharafizad et al. [43] also reported that moderate dosage of salicylic acid (1.2 mM) increased spike length/spike. It has been proposed that zinc spray alleviates drought stress effect on one thousand grain weight [16]. Maleki et al. [46] also stated that, zinc sulfate can decrease negative effects of drought stress on maize grain yield and yield components. A similar protective effect of zinc sulfate was also observed by Malek-Mohammadi et al. [47].
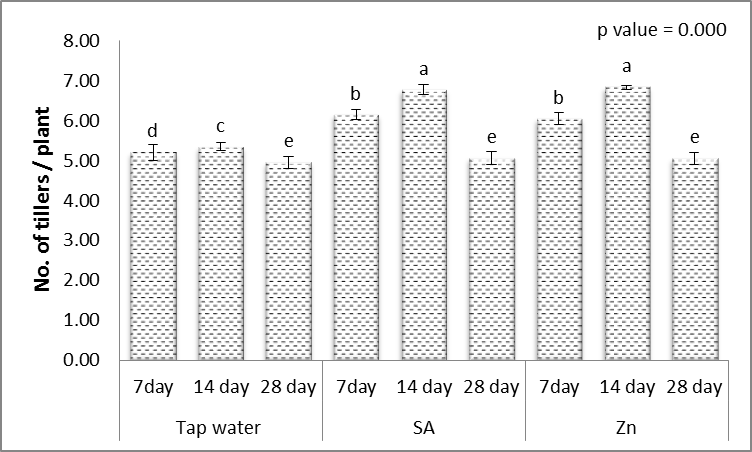 | Figure 1. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on No. of tillers/plant of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
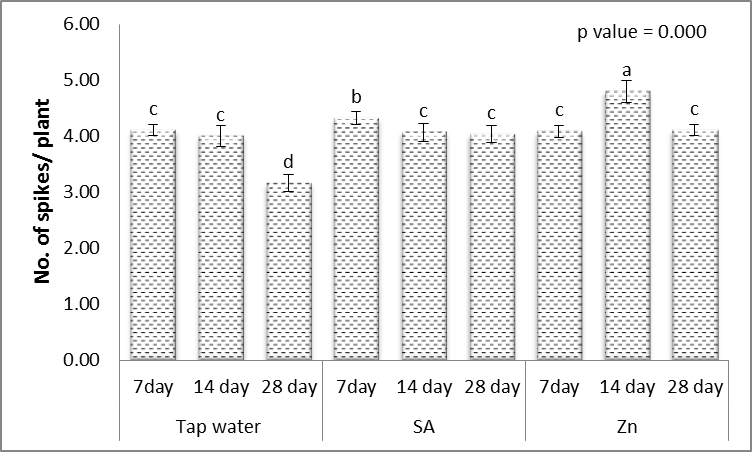 | Figure 2. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on No. of spikes/plant of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
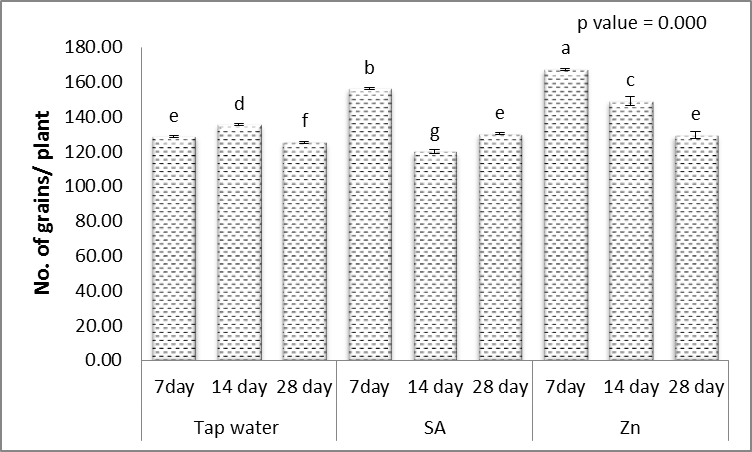 | Figure 3. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on No. of grains/plant of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
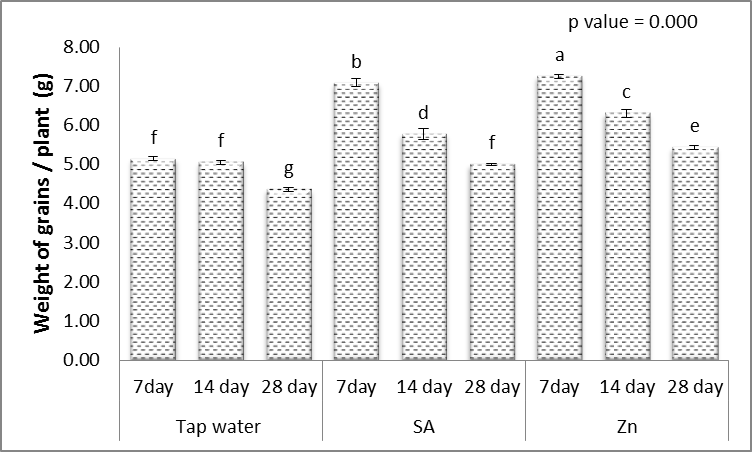 | Figure 4. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on weight. of grains/plant of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
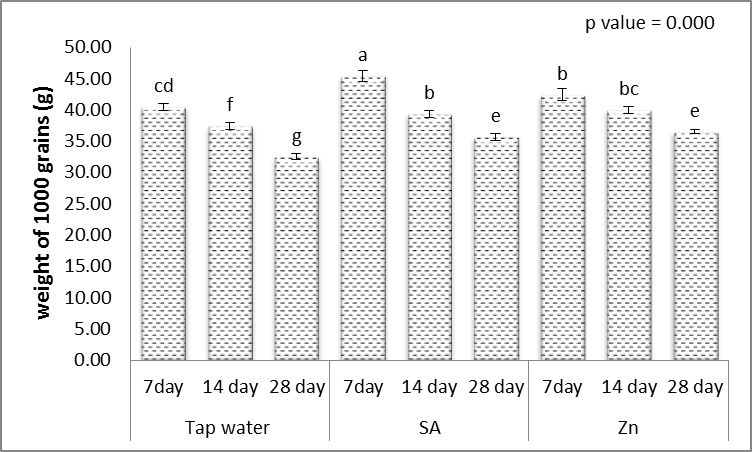 | Figure 5. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on weight of 1000-grains of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.3. Relative Water Content (RWC %)
- Statistical analysis of data for relative water content of leaf demonstrated the effect of different levels at irrigation interval and foliar application was significant on this trait (Fig. 6). With delayed in irrigation, relative water content significantly decreased therefore the highest (31.3%) and lowest (20.8%) RWC were noticed in treatments of irrigation every 14 and 28 days, respectively (Fig. 6). According to the Rahman et al., [48] reports, plants grown under water stress conditions decrease the intracellular water by increasing of osmotic compounds to absorb water from the soil powerfully. It seems there is a direct relationship between the soil moisture content and relative water content of leaf so that reduction in soil moisture and increasing water stress reduces relative water content of leaf. Salicylic acid (SA) treatment markedly alleviated the effect of drought stress and also increased this trait (Fig. 6). There was significant difference SA applied was more effective at all different levels of irrigation interval every 14 and 28 days (Fig. 6). The obtained results were concordant with those of obtained by Singh and Usha [49], who reported that increasing of RWC may be related to the role of SA in accumulation of compatible osmolytes in plants subjected to drought stress. Also, Kabiri et al. [50] reported that, application of SA as foliar spraying recorded the significant values of RWC of Nigella sativa plant grown under drought stress.Foliar application with Zn increased relative water content of wheat leaves, so that the highest level of RWC (4.1%) was obtained in Zn treatment + irrigation interval every 7 days (Fig. 6). To opinion of the Weisany et al. [51] the zinc element have an important role in the regulation of stomatal opening, because this element plays a important role in maintenance of potassium in stomata guard cells and by reducing of leaves water loss, increases the leaf relative water content. Jiang and Huang [52] reported that, difference among leaf relative water contents was significant at Zn levels, so that the plants which received a higher amount of Zn, had more leaf relative moisture. Maintenance of high RWC in drought resistant cultivars has also been reported to be an adaptation to water stress in several crop species.
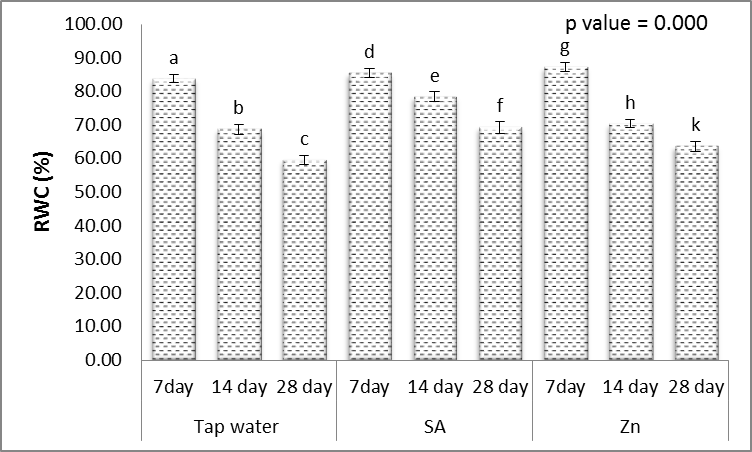 | Figure 6. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on relative water content (RWC %) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.4. Membrane Stability Index (MSI %)
- The results revealed that different levels of irrigation interval significantly decreased the membrane stability index of wheat leaves as compared to unstressed control (Fig. 7). However, SA or Zn application ameliorated the adverse effect of the drought membrane stability index. The most observable indirect effect of drought on plant performance, reduces osmotic potential which results in reduced water availability of plants. These results are in agreement with in agreement with Jaleel et al. [53] in Catharanthus roseus and Yusuf et al. [54] in Brassica juncea. Also, Rao et al. [55] reported that, the membrane stability index of maize plants was, mostly, highly significantly increased in response to the treatment with SA and drought stress.
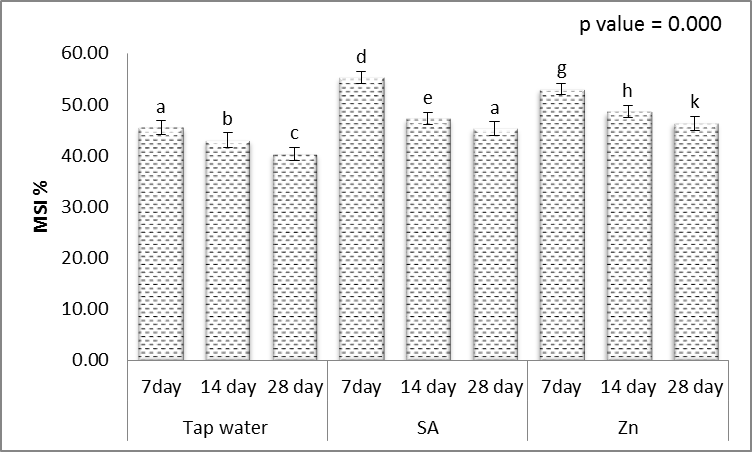 | Figure 7. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on membrane stability index (MSI %) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.5. Electrolyte Leakage (EL)
- The statistical analysis of data indicated that electrolyte leakage significantly affected by irrigation interval every 14 and 28 days (p ≤ 0.01) as well as a foliar application (p ≤ 0.01) (Fig. 8). Mean comparison results showed that the highest electrolyte leakage was recorded by irrigation interval every 28 days and on the contrary, the lowest was seen in the irrigation interval every 14 days (Fig. 8). In drought stress condition, CO2 fixation decreases due the stomata closing, while electron transfer and light reactions continue normality. Under such conditions, NADP found limited amounts to simply accept the electrons and thus oxygen can behave as an electron receptor, inducing the manufacture of reactive oxygen species for example peroxide, superoxide radicals and hydroxyl radicals. Improving the reactive oxygen species cause oxidative injuries in lots of cellular components, for example, proteins, lipids, carbohydrates and nucleic acids and result in increasing of electrolyte leakage and cell membrane peroxidation [52]. In this regard Alexieva et al. [56] reported that drought stress in pea and wheat plant through enlarging of reactive oxygen species production increased electrolyte leakage.Foliar application of SA and Zn with irrigated water every 14 and 28 days decreased electrolyte leakage of leaves (Fig. 8). So that, electrolyte leakage of leaves, indicating cell membrane damage is simply because membrane lipid peroxidation in the presence of reactive oxygen species [52]. According to this, the balance between elimination and production of reactive oxygen species determines the survival of plant systems. Such results are in agreement with those recorded by Yadavi et al [14] in bean who found that application of zinc sulfate at 3 mgL-1 concentration decreased electrolyte leakage in irrigation interval every 50, 75 and 100 mm. Also, Kabiri et al. [50] found that treatment Nigella sativa plants with SA (0, 5, and 10 μM) + interval irrigation at four levels (0, –0.2, –0.4, and –0.6 MPa) caused decrease significant effect on p electrolyte leakage compared to the treated with drought conditions.
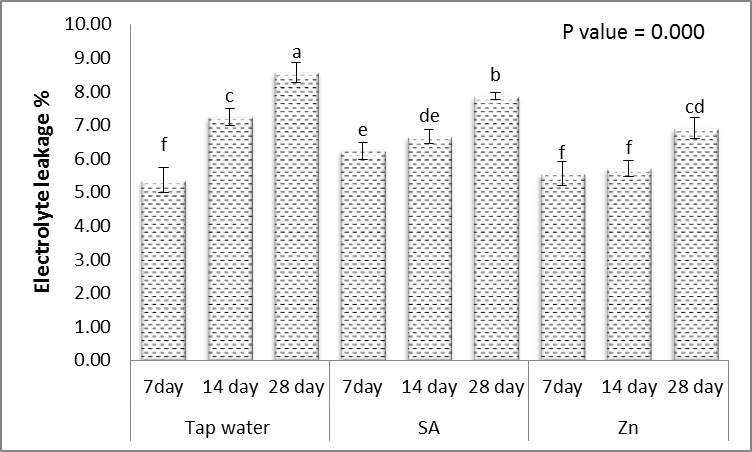 | Figure 8. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on electrolyte leakage (EL %) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.6. Soluble Carbohydrate and Protein Contents
- Results in Figs. (9 & 10) revealed that, the carbohydrate and protein contents in grains of wheat plants were, highly significantly decreased with increasing irrigation level. These results agreed with those of El-Mekawy [57] who mentioned that the effect of irrigation interval on the carbohydrate percentage of black cumin was reduced significantly by decreasing the soil moisture content as a result of increasing the period of irrigation from 2 up to 6 days intervals. Hassan and Ali [58] used three irrigation treatments on Rosmarinus officinalis L. The treatments were 100%, 80% and 60% of the field capacity. They found that carbohydrate percentage increased by deficit irrigation treatments. Rabia et al. [59] found that the carbohydrate percentage of Echinacea purpurea L. significantly decreased as a response to the decrease in irrigation water quantity and reached their minimum value under the lowest irrigation.On the other hand, data presented in Figs. (9 & 10) revealed that, treatment with either SA or Zn resulted in mostly, highly significant increases in the contents of carbohydrate and protein contents in grains of the treated plants, when the irrigation interval every 14 and 28 days. These results are similar to those obtained, by Yadavi et al. [14] and Kabiri et al. [50]. So that salicylic acid caused the increase of protein content in wheat [49]. Improving the activation of nitrate contents and nitrate reductase caused the increase of protein content in SA-treated plants [60]. It appears the character of SA impact on the condition from the hormonal system might lead to protective reactions of plants, acceleration of reparative processes, and also the impact on protein contents [9].The zinc element in stress condition produces an enhancing role in osmotic adjustment process (due to the increase of soluble carbohydrates). Zinc is a vital and occasional consumption element, that have an natural part in protein, and carbohydrate synthesis, cell metabolic process, protection of cell membrane from reactive oxygen species along with other processes connected with adaptation of plants to worry, to ensure that, under drought stress conditions the role of the element is visible like a cause of osmotic regulation, by using intervention within the synthesis of osmotic compounds for compatibility with stress and keep turgor pressure performed their roles [17].
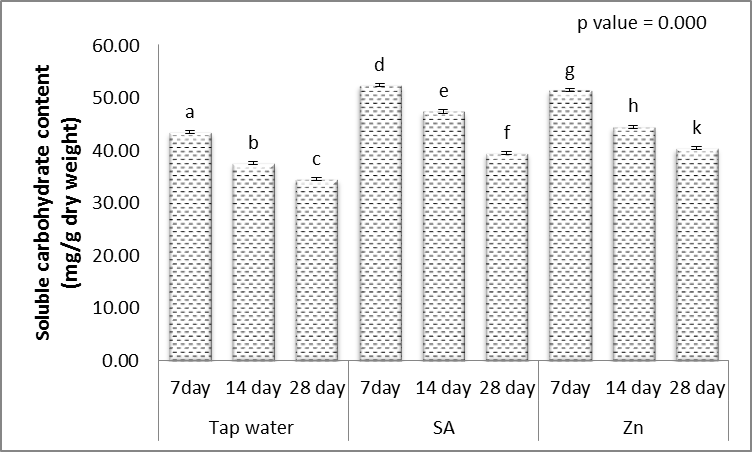 | Figure 9. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on soluble carbohydrate content (mg/g dry weight) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
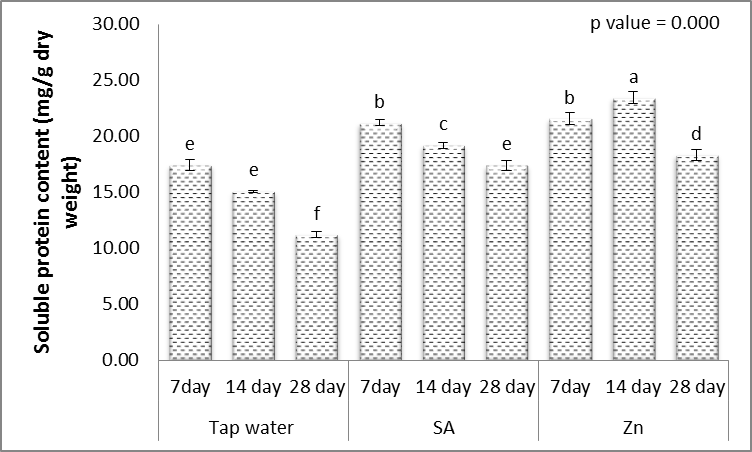 | Figure 10. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on soluble protein content (mg/g dry weight) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.7. Determination of Stress Response Factors
3.7.1. Ascorbic Acid, Glutathione, Lipid Peroxidation, Proline and Phenols
- Hydrophilic antioxidants such as ascorbic acid and glutathione are indispensable components of the antioxidant system which scavenge ROS [61]. The obtained results (Figs. 11-15) showed that, treating wheat plants with different levels of irrigation interval every 14 and 28 days resulted in, significant decrease in the contents of ascorbic acid and glutathione in shoots, while significant increases in the activities of lipid peroxidation (MDA) and proline when compared to unstressed plant.
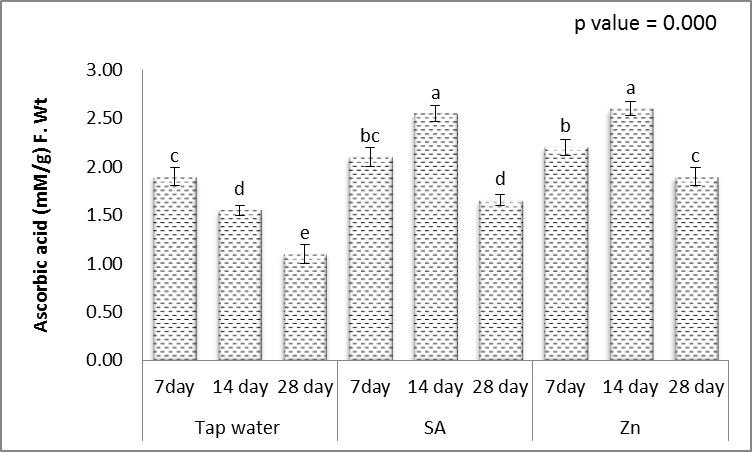 | Figure 11. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on ascorbic acid of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
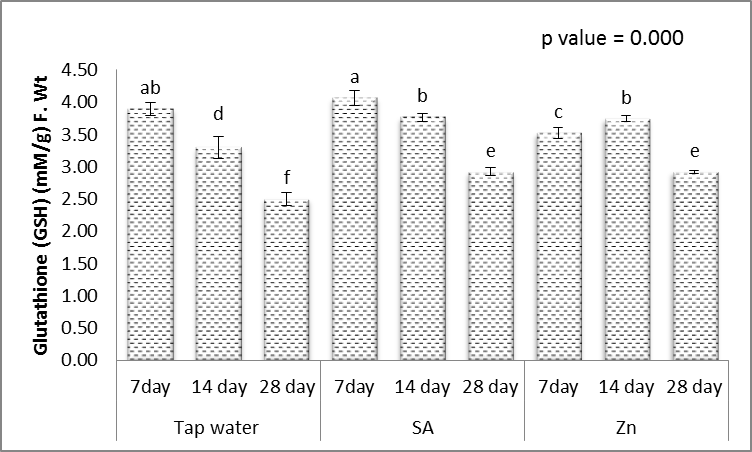 | Figure 12. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on glutathione (GSH) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
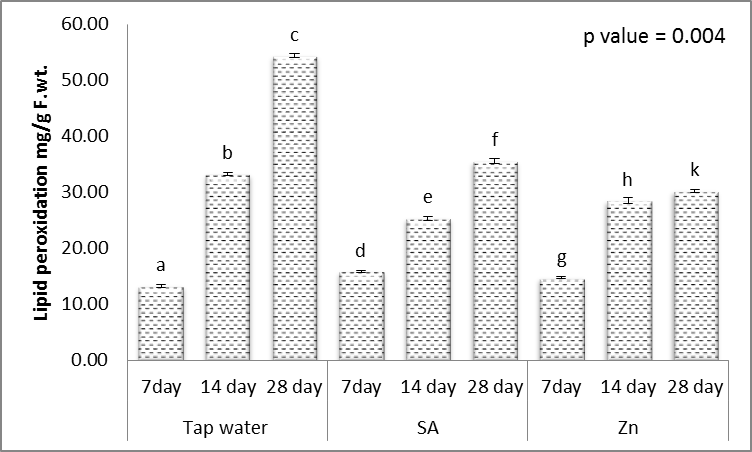 | Figure 13. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on lipid peroxidation (MDA) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
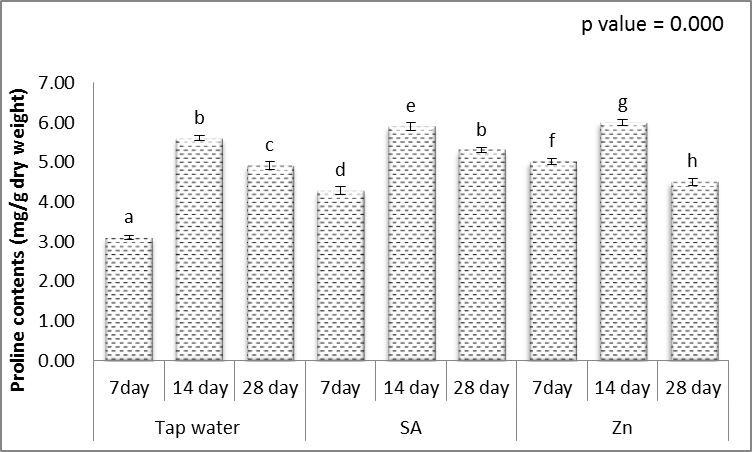 | Figure 14. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on proline contents of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
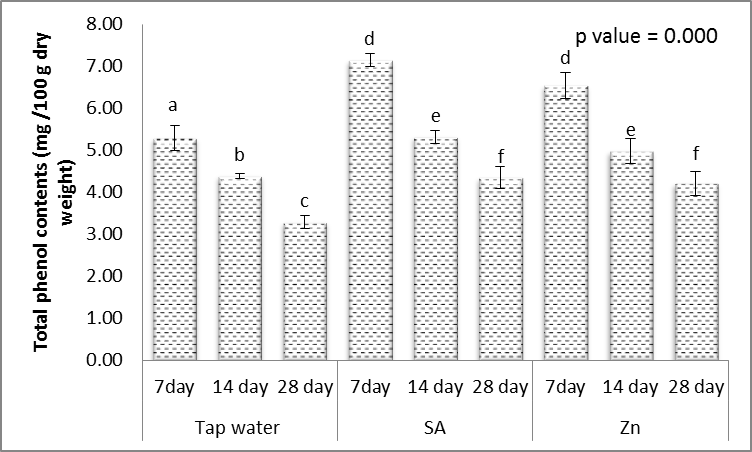 | Figure 15. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on total phenols content (mg/100 g dry weight) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.8. Antioxidant Enzymes
- Tolerance to drought-stress in higher plants correlates to the amount of antioxidant systems and substrates [79]. To combat the results of drought-caused oxidative stress, plants creates a complex mechanism of the antioxidant system. Relatively greater activities of ROS-scavenging enzymes happen to be reported the antioxidant system plays a huge role in plant tolerance against ecological stress.To prevent the effects of drought–generated oxidative stress, plants create a complex mechanism of the antioxidant system. Relatively higher activities of ROS-scavenging enzymes happen to be reported the antioxidant system plays a huge role in plant tolerance against ecological stress. This indicated plants will produce more SOD, POX, CAT, GR and APX enzymes under drought conditions to eliminate the extra ROS in cells. Within this study, SOD, POX, CAT, GR and APX activities elevated markedly within the drought tolerant (Figs. 16-20). This demonstrated that drought-tolerant were efficient scavenger of H2O2, which may result in better protection against H2O2. The SOD detoxifies superoxide anion free radicals (O2-) by forming H2O2 and so the H2O2 could be reduced by POX and CAT [80].
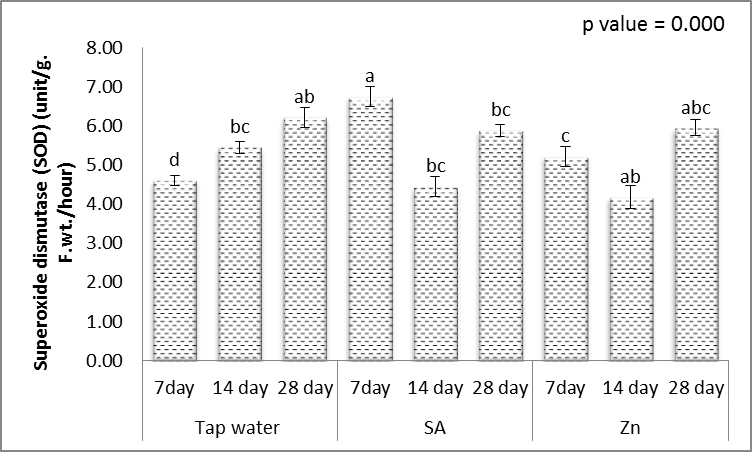 | Figure 16. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on superoxide dismutase (SOD) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
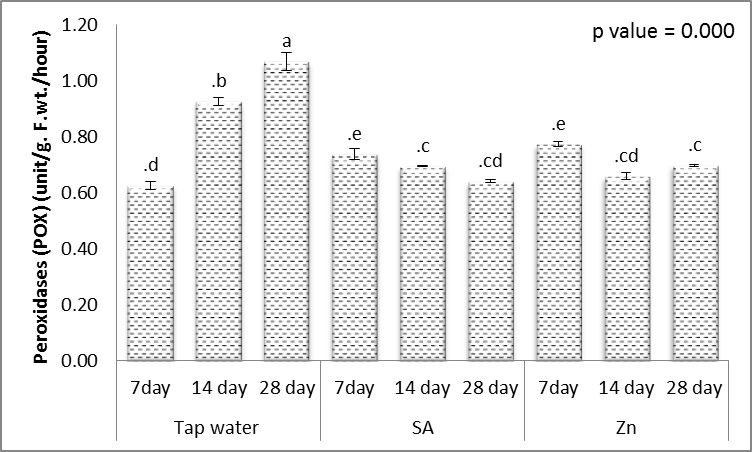 | Figure 17. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on peroxidase (POX) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
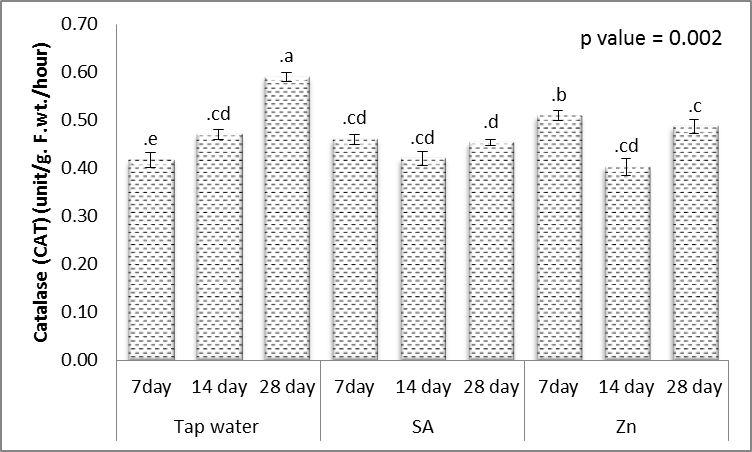 | Figure 18. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on catalase (CAT) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
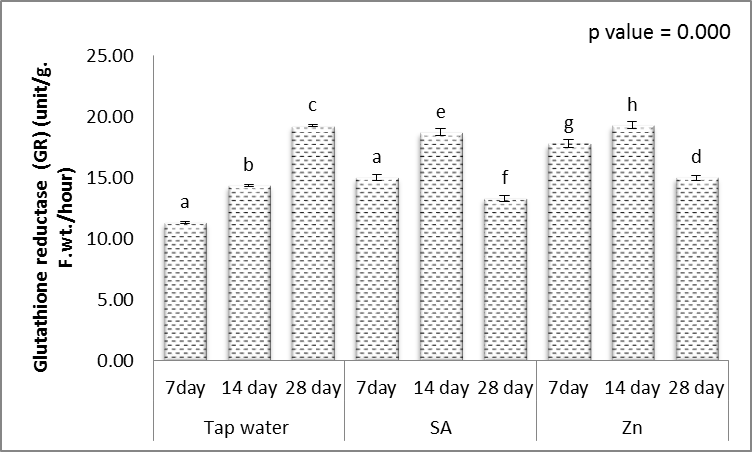 | Figure 19. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on glutathione reductase (GR) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
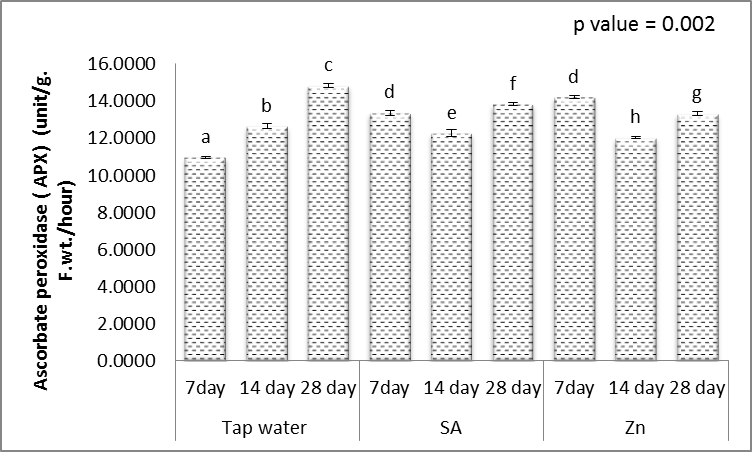 | Figure 20. Effect of salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval on ascorbate peroxidase (APX) of wheat (Triticum aestivum L. var. Giza 168). The bars with the different letters are significantly different at P ≤ 0.05 |
3.9. Correlation
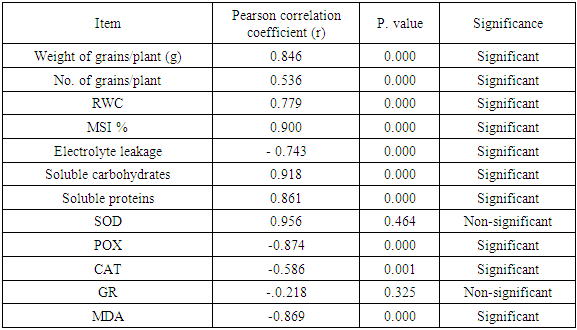
- The result of the correlation analysis under water stress condition showed that weight of 1000-grains and morphology (Weight of grains/plant (g), No. of grains/plant) and biochemical (RWC, MSI, EL, soluble carbohydrates, soluble protein, proline content, SOD, POX, CAT, GR and MDA) parameters had significant correlation (Table 4). There were positive and significant correlation among weight of 1000-grains and morphology, biochemical (RWC, MSI, soluble carbohydrates and soluble protein). In contrast, there were negative and significant correlation between weight of 1000-grains and biochemical (EL, POX, CAT and MDA) parameters. While, non-significant correlation among weight of 1000-grains, SOD and GR. Similar results were reported by Attarbashi et al. [91] and Munir et al. [92] which showed that most of the physiological traits significantly affected the grain yield in wheat crop.
|
3.10. Automatic Linear Modeling and Discriminant Analysis
- Finally, when used the yielded weight as a target; result observed that the accuracy of all data about 96.9%. The effect of target weight of 1000-grains, the wide line is very effective as MSI, then the number of grains/plant, then soluble protein, then MDA and then GSH. Also, the coefficient of target yield weight is the positive blue color wide line which effective as MSI, soluble protein and GSH. While, negative orange color wide line is effective as the number of grains/plant and MDA (Fig. 22). The discriminant analysis that led to the conclusion when foliar application with SA or Zn on wheat plants, we resulted that the SA or Zn is the best treatment to alleviate the stress condition as shown in figure (23).
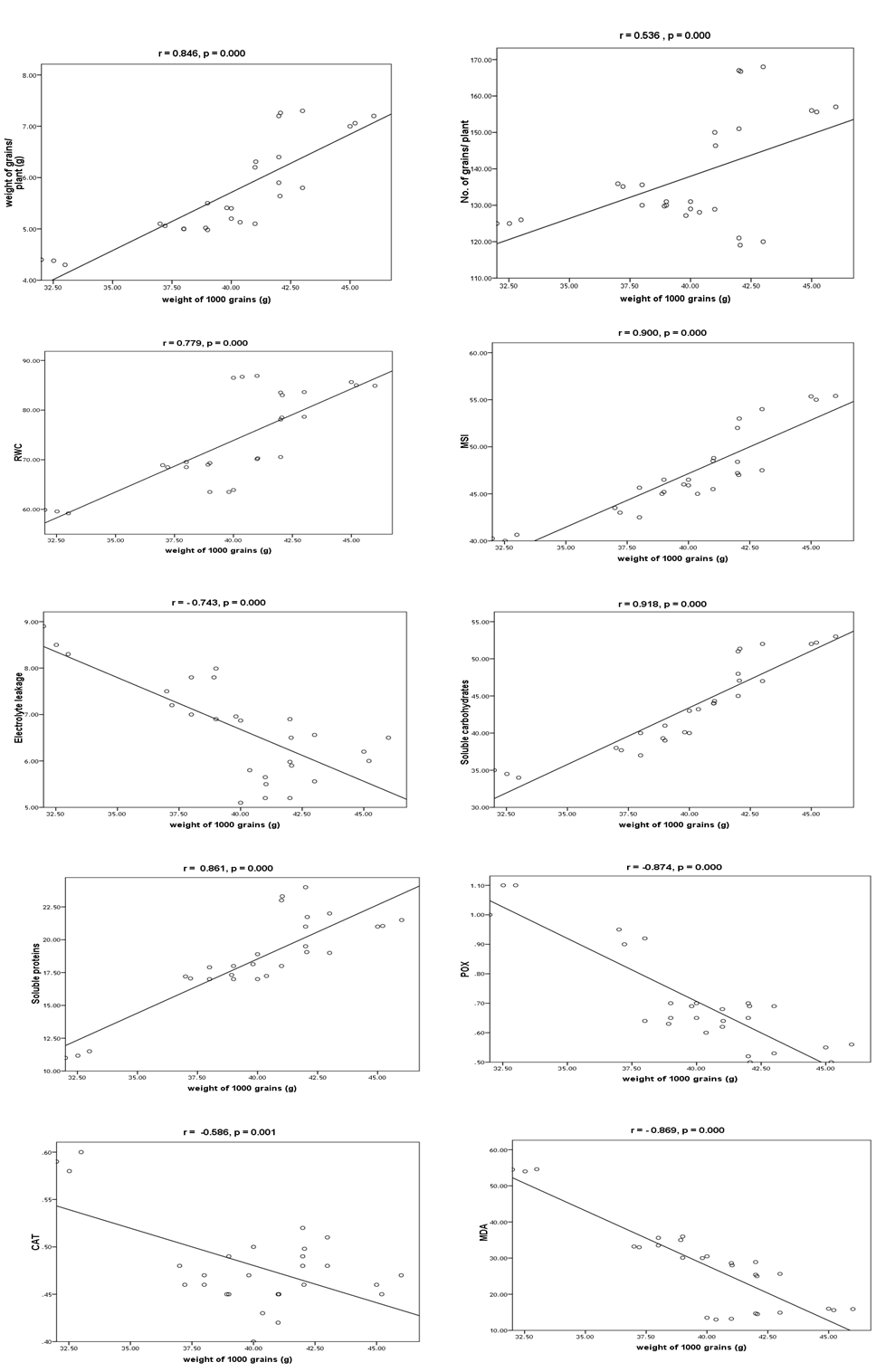 | Figure 21. Linear correlation between weight of 1000-grains and items of morphology, biochemical and their statistical significance |
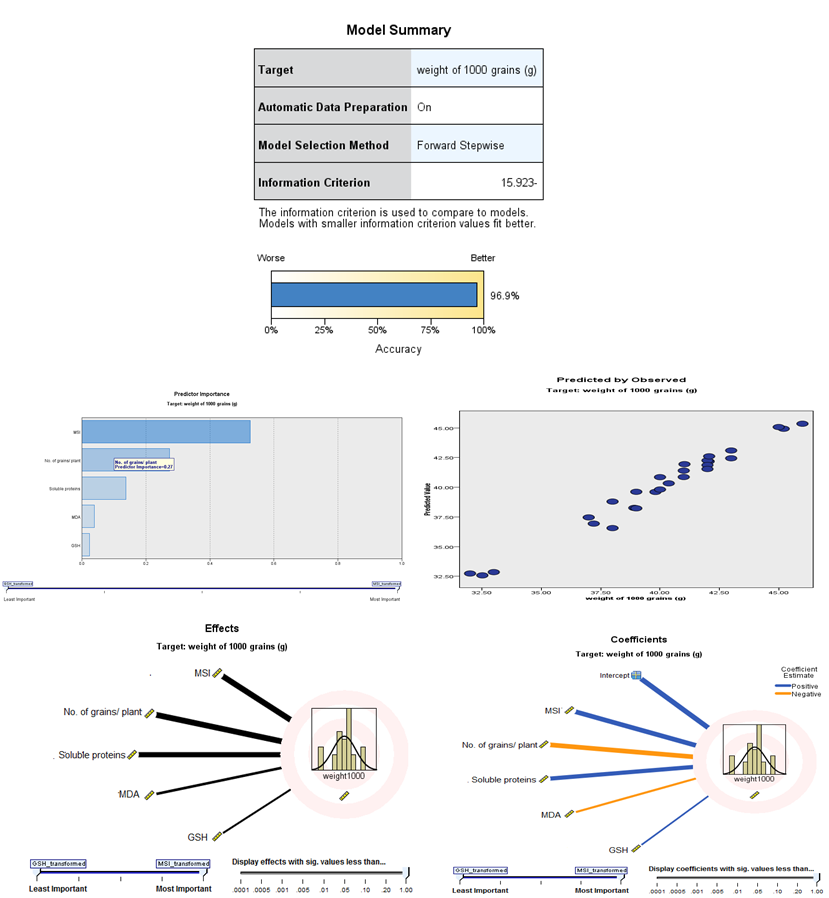 | Figure 22. Automatic linear modeling of the yielded grains of the wheat in response to salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval |
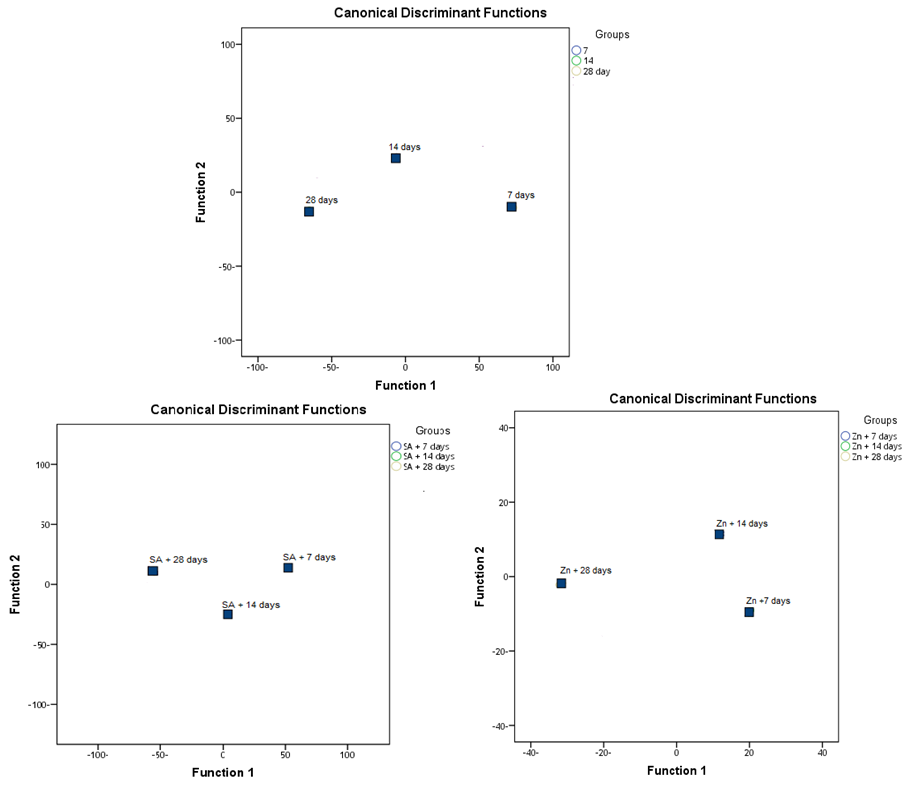 | Figure 23. Discriminant of the yielded grains of the wheat in yielded grains in response to salicylic acid (SA), Zinc (Zn) and different levels of irrigation interval |
4. Conclusions
- In conclusion, water stress negatively affected the growth, yield, biochemical and antioxidant enzymes of the wheat plant. However, application with salicylic acid or zinc application had a beneficial effect on growth and chemical constituents as well as yield quality of wheat plants under different levels of irrigation interval.
References
| [1] | Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; Sfekete, B.M.; Folberth, C.; Foster, I.; Gosling, S.N.; Haddeland, I.; Khabarov, N.; Ludwig, F.; Masaki, Y.; Olin, S.; Rosenzweig, C.; Ruane, A.C.; Satoh, Y.; Schmid, E.; Stacke, T.; Tang, Q. and Wisser, D. (2014): Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci., 111, no. 9, 3239-3244. |
| [2] | Zobayed, S.M.A.; Afreen, F. and Kozai, T. (2007): Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ. Exp. Bot. 59: 109–116. |
| [3] | Selmar, D. (2008): Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforschung 58: 139–144. |
| [4] | Monakhova, O.F. and Chernyadev, I.I. (2002): Protective role of kartolin-4 in wheat plants exposed to soil drought. Applied and Environmental Microbiology, 38: 373–380. |
| [5] | Bukhat, N.M. (2005): Studies in yield and yield associated traits of wheat (Triticum aestivum L.) genotypes under drought conditions. MSc Thesis. Department of Agronomy. Sindh Agriculture University, Tandojam, Pakistan. |
| [6] | El-Kholy, M.A.; Ouda, S.A.; Gaballah, M.S. and Hozayn M. (2005): Predicting the interaction between the effect of anti-transpirant and weather on productivity of wheat plant grown under water stress. Journal of Agronomy. No. 4 p. 75‒82. |
| [7] | Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A. and Fatkhutdinova, D.R. (2003): Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 164: 317–322. |
| [8] | Morris, K.; Mackerness, S.A.H. and Page, T. (2000): Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J., 23, 677–685. |
| [9] | Hayat, S. and Ahmad, A. (eds) (2007): Salicylic Acid: A Plant Hormone. Springer Publ., Dordrecht. |
| [10] | Farooq, M.A.; Wahid, N.; Kobayashi, D.; Fujita, S. and Basra, S.M.A. (2009): Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29, 185–212. |
| [11] | El Tayeb, M. and Ahmed, N.L. (2010): Response of wheat cultivars to drought and salicylic acid. American-Eurasian J. Agron., 3 (1): 1-7. |
| [12] | Fateh, E.; Jiriaii, M.; Shahbazi, S. and Jashni, R. (2012): Effect of salicylic acid and seed weight on germination of wheat (CV. BC ROSHAN) under different levels of osmotic stress. European J. Exp. Biology. 2 (5):1680-1684. |
| [13] | Hashempour, A.; Ghasemzhad, M.; Fotouhi, G. and Sohani, M.M. (2014): The physiological and biochemical response to freezing stress olive plants treated with salicylic acid. Russian J. Plant Physio., 61(4): 443-450. |
| [14] | Yadavi, A.; Aboueshaghi, R.S.; Dehnavi, M.M. and Balouchi, H. (2014): Effect of micronutrıents foliar application on grain qualitative characteristics and some physiological traits of bean (Phaseolus vulgaris L.) under drought stress. Indian J. Fundamental Applied Life Sci., 4(4):124-131. |
| [15] | Zafar, S.; Nasri, M.; Moghadam, H.R.T. and Zahedi, H. (2014): Effect of zinc and sulfur foliar applications on physiological characteristics of sunflower (Helianthus annuus L.) under water deficit stress. Int. J. Biosciences. 5(12):87-96. |
| [16] | Monjezi, F.; Vazini, F. and Hassanzadehdelouei, M. (2013): Effects of iron and zinc spray on yield and yield components of wheat (Triticum aestivum L.) in drought stress. Cercetări Agronomice în Moldova. XLVI (1):153. |
| [17] | Akbari, G.A.; Amirinejad, M.; Baghizadeh, A.; Allahdadi, I. and Shahbazi, M. (2013): Effect of Zn and Fe foliar application on yield, yield components and some physiological traits of cumin (Cuminum cyminum) in dry farming. Int. J. Agro. Plant Prod. 4(12):3231-3237. |
| [18] | Kanani, S.M., Kasraie, P. and Abdi, H. (2013) : Effects of late Season Drought Stress on Grain Yield, Protein, Proline and ABA of Bread Wheat Varieties. Int. J. Agron. Plant Prod. 4 (11): 2943- 2952. |
| [19] | Nelson, D.W. and Sommers, L.E. (1996): In: Sparks, D. L. (ed.), Methods of Soil Analysis (Ed. Am. Soc Agron., Madison, WI.) 961. |
| [20] | Turner, N.C. and Kramer, P.J. (Ed) (1980): Adaptation of Plant to Water and High Temperature Stress. Wiley Interscience Pub, New York, 207-230. |
| [21] | Sairam, R.K. (1994): Effect of moisture stress on physiological activities of two contrasting wheat genotypes, Indian J Exp Biol 3:584-593. |
| [22] | Sullivan, C.Y. and Ross W.M. (1979): Selecting for drought and heat resistance in grain sorghum. In: Mussell H. and Staples R.C.(eds), Stress Physiology in Crop Plants. John Wiley and Sons,New York, pp. 263–281. |
| [23] | Umbriet, W.W.; Burris, R.H.; stauffer, J.F.; Cohen, P.P.; Johsen, W.J.; Lee G.A., Patter, V.R. and Schneicter, W.C. (1969): Manometric techniques, manual describing methods applicable to the studs of tissue metabolism. Burgess publishing Co., U.S.A., pp: 239. |
| [24] | Lowery, O.H.; Rosebrough, N.J.; Farr, A.L. and Randall, R.J. (1951): Protein measurement with the folin reagent. J. Biol. Chem., 193, 265-275. |
| [25] | Jagota, S.K. and Dani, H.M. (1982): A New Calorimetric Technique for the Estimationof Vitamin C Using Folin Phenol Reagent. Analytical Biochemistry 127, 178- 182. |
| [26] | Beutler, E.; Duron, O. and Kelly, B.M. (1963): Improved Method for Determination of Blood Glutathione. Journal of Laboratory and Clinical Medicine, 61, 882-888. |
| [27] | Herna´ndez, J.A. and Almansa, M.S. (2002): Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant, 115: 251–257. |
| [28] | Bates, L.S.; Waldren, R.P. and Teare, I.D. (1973): Rapid determination of free proline for water stress studies. Plant Soil, 39: 205-207. |
| [29] | Dai, J. and Mumper, R.J. (2010): Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules; 15: 7313-52. |
| [30] | Mu Kherjee, S.P. and Choudhuri, M.A. (1983): Implication of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedling. Physiol. Plant, 58: 166-170. |
| [31] | Dhindsa, R.; Plumb-Dhindsa, P. and Thorpe, T. (1981): Leaf senescence correlated permeability, lipid peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot., 32: 93-101. |
| [32] | Bergmeyer, H.U. (1974): Methods of Enzymatic Analysis 1. Second ed. Academic Press, New York. |
| [33] | Chen, Y.; Cao, X.D.; Lu, Y.X. and Wang, R. (2000): Effects of rare earth metal ions and their EDTA complexes on antioxidant enzymes of fish liver. Bull. Environ. Contam. Toxicol., 65: 357- 365. |
| [34] | Karni, L.; Moss, S. and Tel-OR, E. (1984): Glutathione reductase activity in heterocysts and vegetative cells of cyanobacterium Nostoc muscrum. Arch. Microbiol., 140: 215-217. |
| [35] | Snedecor, G.W. and Cochran, W.G. (1973): "Statistical Methods". 6th ed., Iowa State University Press, Iowa, USA. 593. |
| [36] | Härdle, W. and Simar, L. (2007): Applied Multivariate Statistical Analysis. 2nd ed, Springer, 420 pp. |
| [37] | Raza, S.M.A.; Saleem, M.F.; Khan, I.H.; Jamil, M.; Ijaz, M. and Khan, M.A. (2012): Evaluating the drought stress tolerance efficiency of wheat (Triticum aestivum L.) Cultivars. Russian J. Agri. Socio-Economic Sci., 12(2):41-46. |
| [38] | Yan, L. and Shi, Y. (2013): Effect of Drought Stress on Growth and Development in Winter Wheat with Aquasorb-Fertilizer. Adv. Jo. Food Sci. Tech., 5(11): 1502-1504. |
| [39] | Praba, M.L.; Cairns, J.E.; Babu, R.C. and Lafitte, H.R. (2009): Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science195, 30-46. |
| [40] | Anosheh, H.P.; Emam, Y.; Ashraf, M. and Foolad, M.R. (2012): Exogenous application of salicylic acid and chlormequat chloride alleviates negative effects of drought stress in wheat. Advanced Studies in Biology. 4(11):501-520. |
| [41] | Aldesuquy, S.H.; Abbas, M.A.; Abo-Hamed, S.A.; Elhakem, A.H. and Alsokari, S.S. (2012): Glycine betaine and salicylic acid induced modification in productivity of two different cultivars of wheat grown under water stress. J. Stress Physio. Biochem., 8(2): 72-89. |
| [42] | Azimi, M.S.; Daneshian, J.; Sayfazadeh, S. and Zare, S. (2013): Evaluation of Amino Acid and Salicylic Acid application on yield and growth of wheat under water deficit. Int. J. Agri. Crop. Sci. 5 (8): 816-819. |
| [43] | Sharafizad, M.; Naderi, A.; Siadat, S.A.; Sakinejad, T. and Lak, S. (2013): Effect of drought stress and salicylic acid treatment on grain yield, process of grain growth, and some of chemical and morphological traits of Chamran cultivar wheat (Triticum aestivum). Adv. Environ. Biology. 7(11): 3234-3240. |
| [44] | Zamaninejad, M.; Khorasani, S.K.; Moeini, M.J. and Heidarian, A.R. (2013): Effect of salicylic acid on morphological characteristics, yield and yield components of corn (Zea mays L.) under drought condition. European J. Exp. Biology. 3(2):153- 161. |
| [45] | Beigzadeh, S.; Fatahi, K.; Sayedi, A. and Fatahi, F. (2013): Study of the effects of late-season drought stress on yield and yield components of irrigated barley lines within Kermanshah Province Temperate Regions. World Applied Programming. 3(6):226-231. |
| [46] | Maleki, A.; Fazel, S.; Naseri, R.; Rezaei, K. and Heydari, M. (2014): The effect of potassium and zinc sulfate application on grain yield of maize under drought stress conditions. Advances in Environ. Biology. 8(4): 890-893. |
| [47] | Malek-Mohammadi, M.; Maleki, A.; Siaddat, S.A. and Beigzade, M. (2013): The effect of zinc and potassium on the quality yield of wheat under drought stress conditions. I JACS. 6(16):1164- 1170. |
| [48] | Rahman, K.H.; Link, U.; Hocking, W. and Stoddard, F. (2007): Evaluation of physiological traits for improving drought tolerance in faba bean (Vicia Faba L.). Plant and Soil 292 205-217. |
| [49] | Singh, B. and Usha, K. (2003): Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul; 39: 137–41. |
| [50] | Kabiri, R.; Fatemeh, N. and Hassan, F. (2014): Effect of Exogenous Salicylic Acid on Some Physiological Parameters and Alleviation of Drought Stress in Nigella sativa Plant under Hydroponic Culture Plant Protect. Sci. Vol. 50, 2014, No. 1: 43–51. |
| [51] | Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A. and Ghassemi- Golezani, K. (2011): Physiological responses of soybean (Glycine max L.) to zinc application under salinity stress. Australian Journal of Crop Science 5(11) 1441-1447. |
| [52] | Jiang, Y. and Huang, N. (2001): Drought and Heat stress injury to two cool season turf grasses in relation to antioxidant metabolism and lipid peroxidation. Crop Science 41 436-442. |
| [53] | Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R. and Panneerselvam, R. (2007): Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Colloids Surf. B: Biointerfaces, 60: 201-206. |
| [54] | Yusuf, M.; Hasan, S.A.; Ali, B.; Hayat, S.; Fariduddin, Q. and Ahmad, A. (2007): Effect of salicylic acid on salinity induced changes in Brassica juncea. J Integr Plant Biol; 50(9): 1096-1102. |
| [55] | Rao, S. R.; Qayyum, A.; Razzaq, A.; Ahmad, M.; Mahmood, I. and Sher, A. (2012): Role Of Foliar Application Of Salicylic Acid And L-Tryptophan In Drought Tolerance Of Maize. J. Animal and Plant Sciences, 22(3): 768-772. |
| [56] | Alexieva, V.; Sergiev, I.; Mapelli, S. and Karanov, E. (2001): The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat plant. Cell and Environment 24 1337–1344. |
| [57] | El-Mekawy, M.A.M. (2012): Growth and yield of Nigella sativa L. plant influenced by sowing date and irrigation treatments. Amer-Eur J. Agric Environ Sci 12 (4): 499-505. |
| [58] | Hassan, F.A.S. and Ali, E.F. (2013): Impact of different water regimes based on class-A pan on growth, yield and oil content of Coriandrum sativum L. plant. J Saud Soc Agric Sci DOI: 10.1016/j.jssas.2013.05.001. |
| [59] | Rabia, M.M.; Yousef Soha, E. and Khalil Nadia, A.M. (2013): Response of Echinacea purpurea L. To irrigation water regime and biofertilization in sandy soils. World App Sci J 26 (6): 771-782. |
| [60] | Fariduddin, Q.; Hayat, S. and Ahmad A. (2003): Salicylic acid influences net photosynthetic rate, carboxilation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica, 41: 281–284. |
| [61] | Foyer, C.H. and Noctor, G. (2005): Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environment 28, 1056–1071. |
| [62] | Xu, S.; Li, J.; Zhang, X.; Wei, H. and Cui, L. (2006): Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 56: 274-285. |
| [63] | Kumar, K.; Devi, S.S.; Krishnamurthi, K.; Kanade, G.S. and Chakrabarti, T. (2007): Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere 68: 317-322. |
| [64] | André, D.A.N.; José, T.P.; Joaquim, E.F.; Carlos Eduardo, B.D. and Enéas, G.F. (2006): Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 56: 87-94. |
| [65] | Huseynova, I.M. (2012): Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. Biochimicaet Biophysica Acta-Bioenergetics 1817, 1516–1523. |
| [66] | Naureen, G. and Naqvi, F.N. (2010): Salt Tolerance Classification in Wheat Genotypes Using Reducing Sugar Accumulation and Growth Characteristic. Emirates Journal of Food and Agriculture, 22: 308-317. |
| [67] | Ezzat-Ollah, E.; Shakiba, M.R.; Mahboob, S.A.; Hoshang, A. and Mahmood, T. (2007): Water stress, antioxidant enzyme activity and lipid peroxidation in wheat seedling. Int. J. Food Agric. Environ., 5: 149–153. |
| [68] | Hong, Z.; Lakkineni, K.; Zhang, Z. and Verma, D.P.S. (2000): Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 122: 1129-1136. |
| [69] | Ashraf, M. and Foolad, M.R. (2007): Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59: 206-216. |
| [70] | Syvacy, A. and Sokmen, M. (2004): Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.). Plant Growth Regulation, 44: 251–256. |
| [71] | Sakihama, Y.; Cohen, M.F.; Grace, S.C. and Yamasaki, H. (2002): Plant phenolics antioxidant and proxidant activity: phenolics-induced oxidative damage mediated by metal in plants. Toxicology, 177: 67–80. |
| [72] | Delavari, P.M.; Baghizadeh, A.; Enteshari, S.H.; Kalantari, K.H.M.; Yazdanpanah, A. and Mousavi, E.A. (2010): The Effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicucm under salinity stress. Australian Journal of Basic and Applied Sciences 4(10), 4832-4845. |
| [73] | Agarwal, S.; Sairam, R.K.; Srivasta, G.C. and Meena, R.C. (2005): Changes in antioxidant enzymes activity and oxidative stress by Abscisic acid and salicylic acid in wheat genotypes. Biol Plant 49(4), 541-550. |
| [74] | Yazdanpanah, S.; Baghizadeh, A. and Abbassi, F. (2011): The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. African Journal of Agricultural Research 6(4), 798-807. |
| [75] | Baghizadeh, A.; Ghorbanli, M.; Haj, M.R.M. and Mozafarih, H. (2009): Evaluation of interaction effect of drought stress with ascorbate and salicylic acid on some of physiological and biochemical parameters in Okra (Hibiscus esculentus L.). Research J. of Biological Sciences, 4(4):380-387. |
| [76] | Bartels, D. and Sunkar, R. (2005): Drought and salt tolerance in plants. Crit. Rev. Plant Sci., 24: 23-58. |
| [77] | Metraux, J.P. (2002): Recent breakthroughs in the study of salicylic acid biosynthesis. Trends Plant Sci., 7: 332-334. |
| [78] | André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Alvarado, C.A.; Nomberto, A.G.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y. and Evers, D. (2009): Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry, 70: 1107– 1116. |
| [79] | Athar, H., Khan, A. and Ashraf, M. (2008): Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 63: 224–231. |
| [80] | Hasheminasab, H.; Assad, M.T.; Aliakbari, A. and Sahhafi, R. (2012): Influence of drought stress on oxidative damage and antioxidant defense systems in tolerant and susceptible wheat genotypes. J. Agric. Sci. 4(8): 20-30. |
| [81] | Omar, A.A. (2012): Impact of drought stress on germination and seedling growth parameters of some wheat cultivars. Life Sci. J. 9(1): 590-598. |
| [82] | Mohammadkhani, N. and Heidari, R. (2008): Drought induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 3(3): 448-453. |
| [83] | Sairam, R.K. and Srivastava, G.C. (2001): Water stress tolerance of wheat (Triticum aestivum L.): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop Sci. 186: 63-70. |
| [84] | Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.H. and Ding, R.X. (2003): Exogenous Silicon (Si) Increases Antioxidant Enzyme Activity and Reduces Lipid Peroxidation in Roots of Salt-Stressed Barley (Hordeum vulgare L.). Journal of Plant Physiology, 160, 1157-1164. |
| [85] | Najami, N.; Tibor, J.; Barriah, W.; Kayam, G.; Moshe, T.; Guy, M. and Volokita, M. (2008): Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol Genet Genom 279: 171-182. |
| [86] | Pan, Y.; Wu, L.J. and Yu, Z.L. (2006): Effect of Salt and Drought Stress on Antioxidant Enzymes Activities and SOD Isoenzymes of Liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regulation, 49, 157-165. |
| [87] | Habibi, G. (2012): Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biologica Szegediensis 56(1):57-63. |
| [88] | Wójcik, P. (2004): Uptake of mineral nutrients from foliar fertilization. J. Fruit Ornam. Plant Res. (special ed) 12, 201–218. |
| [89] | Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A. and Mora, M.L. (2010): Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Bio. 156, 297–307. |
| [90] | Xu, H.; Biswas, D.K.; Li, W.D.; Chen, S.B.; Zhang, S.B.; Jiang, G.M. and Li, Y.G. (2007): Photosynthesis and yield responses of ozone-polluted winter wheat to drought. Photosynthetica. 45, 582–588. |
| [91] | Attarbashi, M.R.; Galeshi, S.; Soltni, A. and Zinali, E. (2002): Relationship of phenology and physiological traits with grain yield in wheat under rain-fed conditions. Iranian J. Agric. Sci. 33: 21-28. |
| [92] | Munir, M.; Chowdhry, M.A. and Malik, T.A. (2007): Correlation studies among yield and its components in bread wheat under drought conditions. Int. J. Agric. Biol. 9: 287-290. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML